QUESTION 2 Based on the following synthetic route, cycloalkene J could be used to generate a cyclised ester, N. H3C CH3 ? ELOH H3C ČH3 H3C ČH3 HO. H*,A Cycloalkene K L NABH, Hydroxy derivative M Intramolecular esterification Ester N a) Cycloalkene J has to be initially cleaved to give compound K. State the appropriate reagents and reaction conditions for the cleavage of J. b) Prior to undergoing intramolecular esterification compound K has to be converted to N-DU Duuu thu
QUESTION 2 Based on the following synthetic route, cycloalkene J could be used to generate a cyclised ester, N. H3C CH3 ? ELOH H3C ČH3 H3C ČH3 HO. H*,A Cycloalkene K L NABH, Hydroxy derivative M Intramolecular esterification Ester N a) Cycloalkene J has to be initially cleaved to give compound K. State the appropriate reagents and reaction conditions for the cleavage of J. b) Prior to undergoing intramolecular esterification compound K has to be converted to N-DU Duuu thu
Chapter10: Organohalides
Section10.2: Preparing Alkyl Halides From Alkanes: Radical Halogenation
Problem 4P: Taking the relative reactivities of 1°, 2°, and 3° hydrogen atoms into account, what product(s)...
Related questions
Question
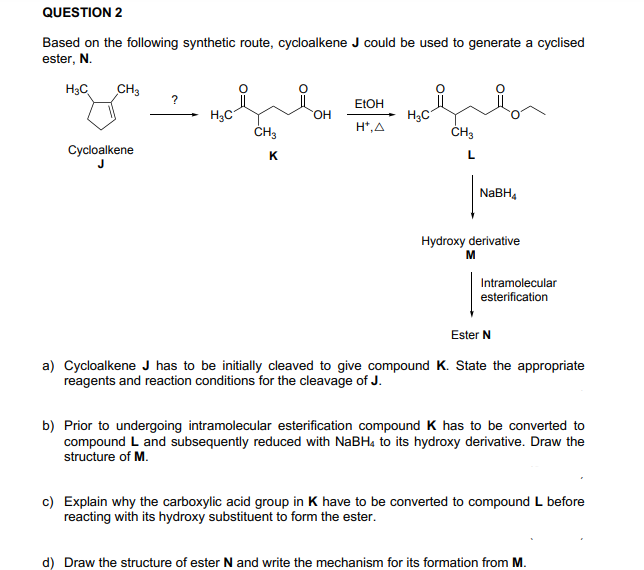
Transcribed Image Text:QUESTION 2
Based on the following synthetic route, cycloalkene J could be used to generate a cyclised
ester, N.
H3C
CH3
?
ELOH
H3C
ČH3
H3C
ČH3
HO.
H*,A
Cycloalkene
K
L
NABH,
Hydroxy derivative
M
Intramolecular
esterification
Ester N
a) Cycloalkene J has to be initially cleaved to give compound K. State the appropriate
reagents and reaction conditions for the cleavage of J.
b) Prior to undergoing intramolecular esterification compound K has to be converted to
compound L and subsequently reduced with NABH4 to its hydroxy derivative. Draw the
structure of M.
c) Explain why the carboxylic acid group in K have to be converted to compound L before
reacting with its hydroxy substituent to form the ester.
d) Draw the structure of ester N and write the mechanism for its formation from M.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

