Question 2. Let us consider a hypothetical Atom with the following list of energy levels: E1 = 1.5 eV E2 = 3.5 eV E3 = 4.8 eV E4 = 5.6 eV a) Compute the energy of ALL the spectral lines in the ABSORPTION SPECTRUM and list the corresponding "colors" b) Compute the energy of ALL the spectral lines in the EMISSION SPECTRUM and list the corresponding "colors" Color violet blue Wavelength Frequency Photon energy (nm) (THz) 380-450 670-790 450-485 620-670 485-500 600-620 500-565 530-600 565-590 510-530 480-510 400-480 cyan green yellow orange 590-625 red 625-750 (ev) 2.75-3.26 2.56-2.75 2.48-2.56 2.19-2.48 2.10-2.19 1.98-2.10 1.65-1.98
Question 2. Let us consider a hypothetical Atom with the following list of energy levels: E1 = 1.5 eV E2 = 3.5 eV E3 = 4.8 eV E4 = 5.6 eV a) Compute the energy of ALL the spectral lines in the ABSORPTION SPECTRUM and list the corresponding "colors" b) Compute the energy of ALL the spectral lines in the EMISSION SPECTRUM and list the corresponding "colors" Color violet blue Wavelength Frequency Photon energy (nm) (THz) 380-450 670-790 450-485 620-670 485-500 600-620 500-565 530-600 565-590 510-530 480-510 400-480 cyan green yellow orange 590-625 red 625-750 (ev) 2.75-3.26 2.56-2.75 2.48-2.56 2.19-2.48 2.10-2.19 1.98-2.10 1.65-1.98
Modern Physics
3rd Edition
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Chapter4: The Particle Nature Of Matter
Section: Chapter Questions
Problem 19P
Related questions
Question
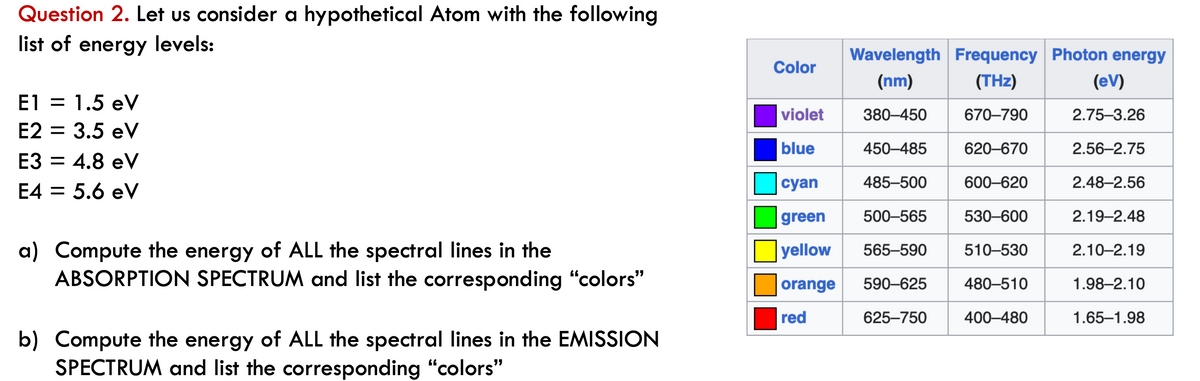
Transcribed Image Text:Question 2. Let us consider a hypothetical Atom with the following
list of energy levels:
E1 = 1.5 eV
E2 = 3.5 eV
E3 = 4.8 eV
E4 = 5.6 eV
a) Compute the energy of ALL the spectral lines in the
ABSORPTION SPECTRUM and list the corresponding "colors"
b) Compute the energy of ALL the spectral lines in the EMISSION
SPECTRUM and list the corresponding "colors"
Wavelength Frequency Photon energy
(nm)
(THz)
380-450
670-790
450-485
620-670
cyan
485-500
600-620
green
500-565
530-600
yellow
565-590
510-530
orange 590-625
480-510
red
625-750
400-480
Color
violet
blue
(eV)
2.75-3.26
2.56-2.75
2.48-2.56
2.19-2.48
2.10-2.19
1.98-2.10
1.65-1.98
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Foundations of Astronomy (MindTap Course List)
Physics
ISBN:
9781337399920
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Foundations of Astronomy (MindTap Course List)
Physics
ISBN:
9781337399920
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning

Stars and Galaxies (MindTap Course List)
Physics
ISBN:
9781337399944
Author:
Michael A. Seeds
Publisher:
Cengage Learning

