QUESTION 21 The Arrhenius equation is k = Ae -Ea/RT The slope of a plot of Ink vs. 1/T is equal to O -k O E /R O A O Ea QUESTION 22 Chlorine dioxide reacts in basic water to form chlorite and chlorate according to the following chemical equation: 2CIO 2(aq) + 20H (aq) CIO 2 (aq) + CIO 3 (aq) + H 20(1) A Linotic otudu of shin ronntionunder cortain set of oondi+ionevieldod the dnte below Click Save and Submit to save and submit. Click Save All Answers to save all answers.
QUESTION 21 The Arrhenius equation is k = Ae -Ea/RT The slope of a plot of Ink vs. 1/T is equal to O -k O E /R O A O Ea QUESTION 22 Chlorine dioxide reacts in basic water to form chlorite and chlorate according to the following chemical equation: 2CIO 2(aq) + 20H (aq) CIO 2 (aq) + CIO 3 (aq) + H 20(1) A Linotic otudu of shin ronntionunder cortain set of oondi+ionevieldod the dnte below Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Chapter7: Neutralization Titrations And Graphical Representations
Section: Chapter Questions
Problem 1P
Related questions
Question
100%
Question 21
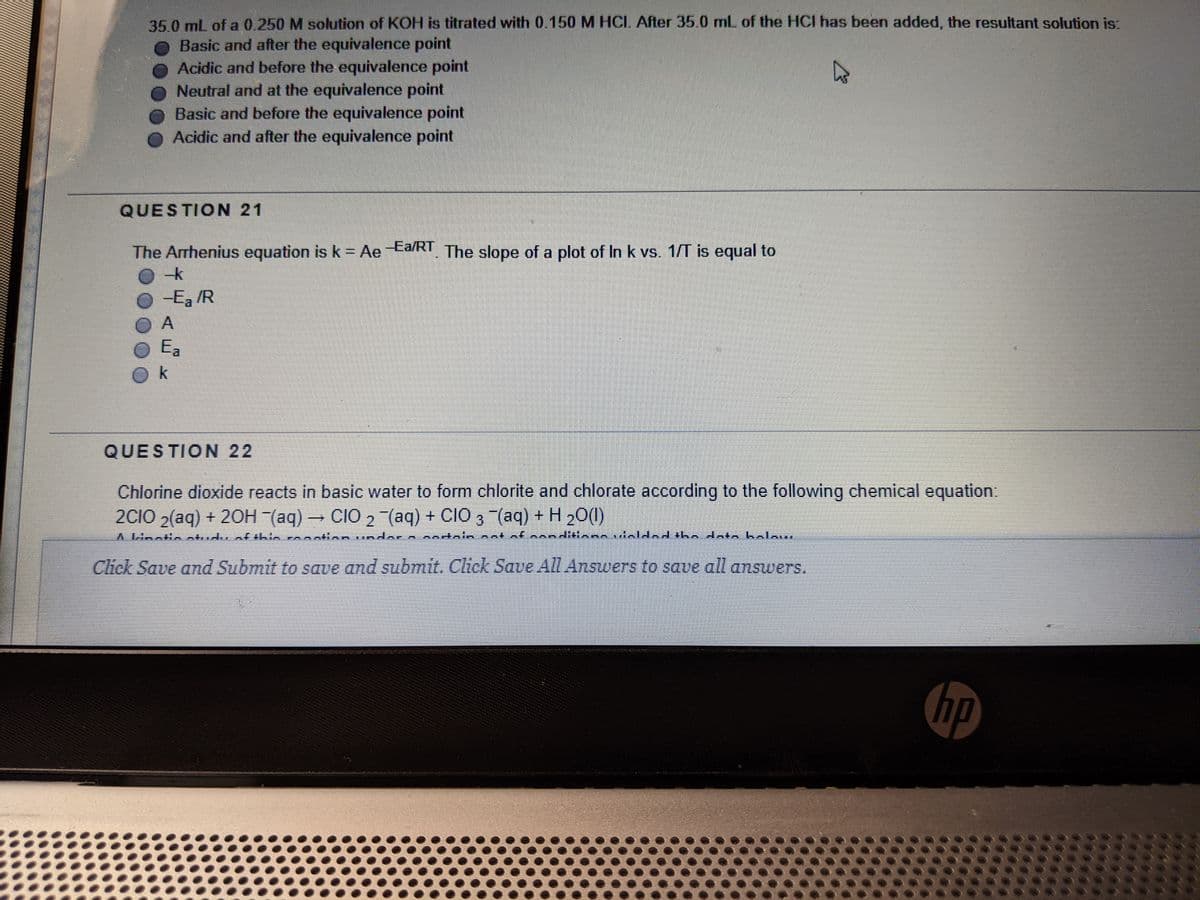
Transcribed Image Text:35.0 mL of a 0.250 M solution of KOH is titrated with 0.150 M HCI After 35.0 mL of the HCI has been added, the resultant solution is:
Basic and after the equivalence point
O Acidic and before the equivalence point
Neutral and at the equivalence point
Basic and before the equivalence point
O Acidic and after the equivalence point
QUESTION 21
etaRI The slope of a plot of In k vs. 1/T is equal to
The Arrhenius equation is k= Ae
O-K
-Ea /R
O A
O E,
QUESTION 22
Chlorine dioxide reacts in basic water to form chlorite and chlorate according to the following chemical equation:
2CIO 2(aq) + 20H (aq) CIO 2 (aq) + CIO 3 (aq) + H 20(1I)
eontion undor
券
Click Save and Submit to save and submit. Chck Save 4ll Answers to save all ansuoers.
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning