Suppose a 0.14 M aqueous solution of oxalic acid (H₂C₂O) is prepared. Calculate the equilibrium molarity of CO You'll find information on the properties of oxalic acid in the ALEKS Data resource. Round your answer to 2 significant digits. M OP
Suppose a 0.14 M aqueous solution of oxalic acid (H₂C₂O) is prepared. Calculate the equilibrium molarity of CO You'll find information on the properties of oxalic acid in the ALEKS Data resource. Round your answer to 2 significant digits. M OP
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 25P
Related questions
Question
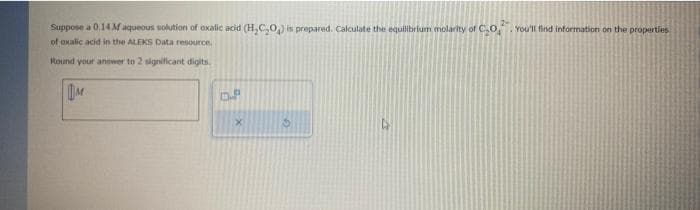
Transcribed Image Text:Suppose a 0.14 M aqueous solution of oxalic acid (H₂C₂O₂) is prepared. Calculate the equilibrium molarity of CO,. You'll find information on the properties.
of oxalic acid in the ALEKS Data resource.
Round your answer to 2 significant digits.
M
D.P
X
S
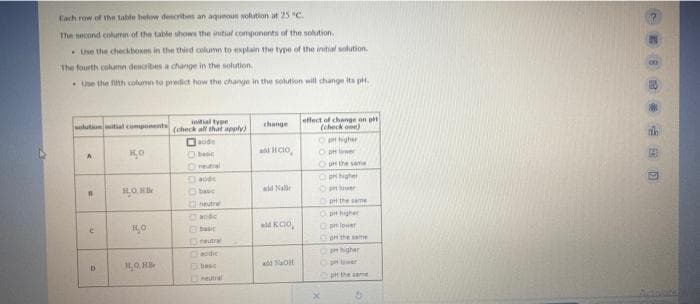
Transcribed Image Text:Each row of the table below describes an aqueous solution at 25 °C.
The second column of the table shows the initial components of the solution.
Use the checkboxes in the third column to explain the type of the initial solution.
The fourth column describes a change in the solution.
Use the fifth column to predict how the change in the solution will change its pH.
initial type
solution witial components (check all that apply)
ade
besc
neutral
ade
basic
neutral
A
.
n
D
H₂O
H.O.H
RO
10.H
Candic
basic
neutral
aodic
bas
change
add HCIO,
add all
KCIO,
add NaOH
effect of change on pit
(check one)
Op higher
Op liner
Op the same
pH highet
x
power
pH the same
higher
power
on the same
higher
theme
80
180
n
141
Acthuta
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning