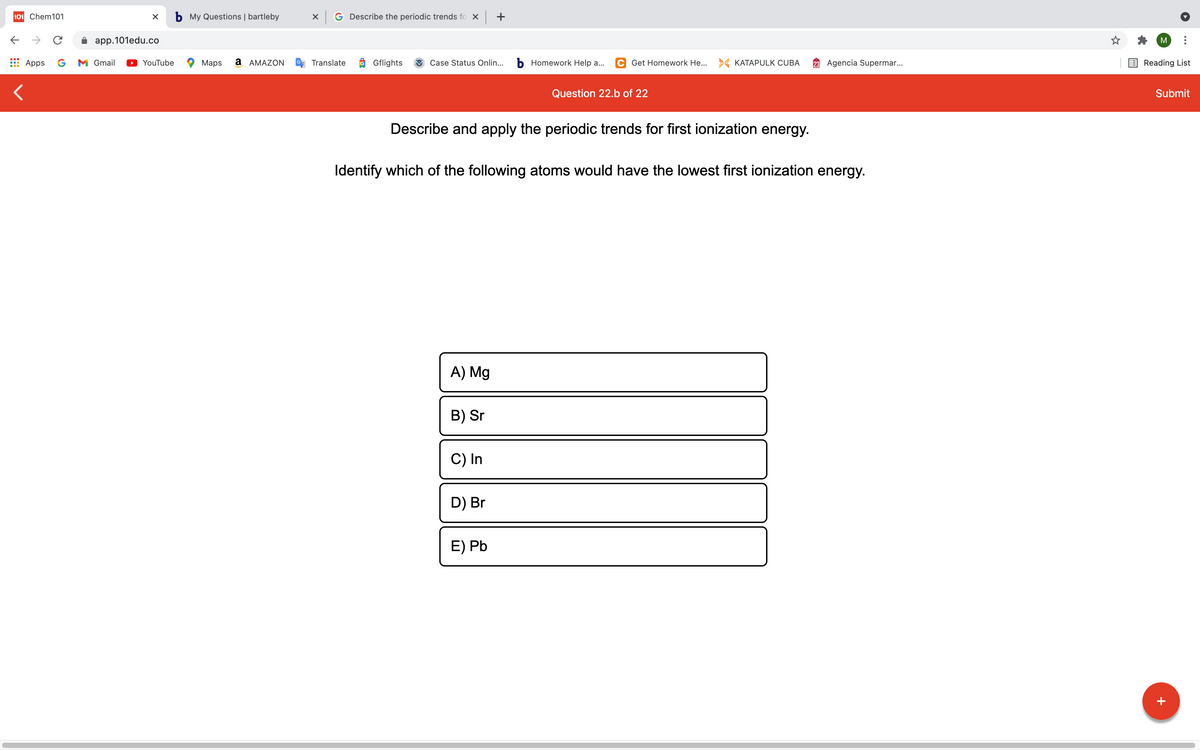
Question 22.b of 22 Describe and apply the periodic trends for first ionization energy. Identify which of the following atoms would have the lowest first ionization energy. A) Mg B) Sr C) In D) Br E) Pb
Question 22.b of 22 Describe and apply the periodic trends for first ionization energy. Identify which of the following atoms would have the lowest first ionization energy. A) Mg B) Sr C) In D) Br E) Pb
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 102QRT
Related questions
Question
Transcribed Image Text:101 Chem101
b My Questions | bartleby
G Describe the periodic trends fo x +
->
app.101edu.co
M
Apps
G
M Gmail
YouTube
Maps
a AMAZON
Translate
Gflights
Case Status Onlin...
b Homework Help a...
C Get Homework He... KATAPULK CUBA
23 Agencia Supermar..
Reading List
Question 22.b of 22
Submit
Describe and apply the periodic trends for first ionization energy.
Identify which of the following atoms would have the lowest first ionization energy.
A) Mg
B) Sr
C) In
D) Br
E) Pb
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning