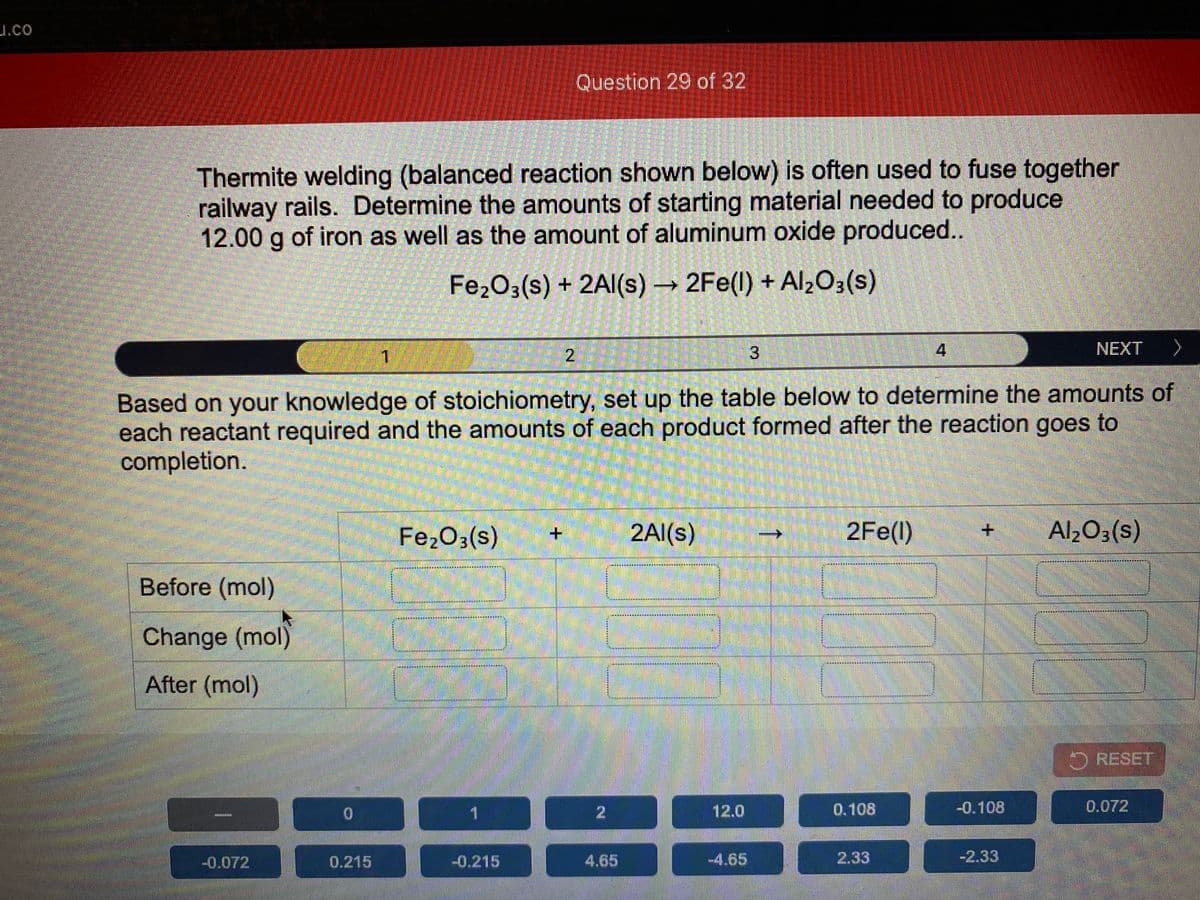
Question 29 of 32 Thermite welding (balanced reaction shown below) is often used to fuse together railway rails. Determine the amounts of starting material needed to produce 12.00 g of iron as well as the amount of aluminum oxide produced.. Fe,O3(s) + 2Al(s) → 2Fe(l) + Al,O3(s) NEXT 1 Based on your knowledge of stoichiometry, set up the table below to determine the amounts of each reactant required and the amounts of each product formed after the reaction goes to completion. Fe2O3(s) 2Al(s) 2Fe(1) Al2O3(s) + + Before (mol) Change (mol) After (mol) O RESET 1. 12.0 0.108 -0.108 0.072 -0.072 0.215 -0.215 4.65 -4.65 2.33 -2.33
Question 29 of 32 Thermite welding (balanced reaction shown below) is often used to fuse together railway rails. Determine the amounts of starting material needed to produce 12.00 g of iron as well as the amount of aluminum oxide produced.. Fe,O3(s) + 2Al(s) → 2Fe(l) + Al,O3(s) NEXT 1 Based on your knowledge of stoichiometry, set up the table below to determine the amounts of each reactant required and the amounts of each product formed after the reaction goes to completion. Fe2O3(s) 2Al(s) 2Fe(1) Al2O3(s) + + Before (mol) Change (mol) After (mol) O RESET 1. 12.0 0.108 -0.108 0.072 -0.072 0.215 -0.215 4.65 -4.65 2.33 -2.33
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 7RQ: Consider the hypothetical reaction between A2 and AB pictured below. What is the balanced equation?...
Related questions
Question
Transcribed Image Text:J.co
Question 29 of 32
Thermite welding (balanced reaction shown below) is often used to fuse together
railway rails. Determine the amounts of starting material needed to produce
12.00 g of iron as well as the amount of aluminum oxide produced..
Fe2O3(s) + 2Al(s) 2Fe(l) + Alz03(s)
1
2
4
NEXT
へ
Based on your knowledge of stoichiometry, set up the table below to determine the amounts of
each reactant required and the amounts of each product formed after the reaction goes to
completion.
Fe203(s)
2Al(s)
2Fe(l)
Al203(s)
其其 就其其其 * 其 K其
利 丼 利其
Before (mol)
Change (mol)
材其 算
After (mol)
O RESET
1
12.0
0.108
-0.108
0.072
-0.072
0.215
-0.215
4.65
-4.65
2.33
-2.33
3.
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co