QUESTION 4 When we use detector tube and the measured concentration is 500 ppm, what is the range of actual concentration? Hint Detector tubes are considered accurate within + 25% O a. 475 to 525 ppm. O b. 450 to 550 ppm Oc 375 to 625 ppm. d. None of the above
QUESTION 4 When we use detector tube and the measured concentration is 500 ppm, what is the range of actual concentration? Hint Detector tubes are considered accurate within + 25% O a. 475 to 525 ppm. O b. 450 to 550 ppm Oc 375 to 625 ppm. d. None of the above
Chapter4: Least-squares And Calibration Methods
Section: Chapter Questions
Problem 9P
Related questions
Question
100%
2
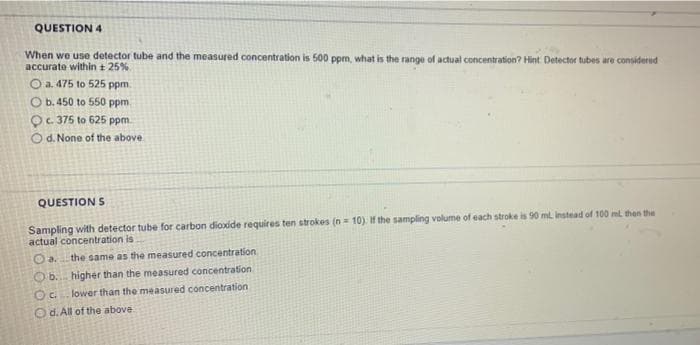
Transcribed Image Text:QUESTION 4
When we use detector tube and the measured concentration is 500 ppm, what is the range of actual concentration? Hint Detector tubes are considered
accurate within + 25%
O a. 475 to 525 ppm.
O b. 450 to 550 ppm.
Oc. 375 to 625 ppm.
Od. None of the above
QUESTION S
Sampling with detector tube for carbon dioxide requires ten strokes (n= 10) if the sampling volume of each stroke is 90 ml instead of 100 ml then the
actual concentration is
O a.
Ob. higher than the measured concentration.
OC. lower than the measured concentration
the same as the measured concentration
O d. All of the above
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning