Question 47 Listen Sulfa drugs, shown below can be used to treat bacteria infections: R-S-NH₂ A Sulfonamide A) If sulfa binds at a site other than the active site, is this a competitive or noncompetitive inhibitor? (covalent, ionic 2) It is irreversible, so the bond formed between the enzyme and antibiotic is likely. or hydrogen bond)? 3) Is the portion of sulfa shown polar or nonpolar? 4) What is a possible amino acid at the binding site of sulfa?
Question 47 Listen Sulfa drugs, shown below can be used to treat bacteria infections: R-S-NH₂ A Sulfonamide A) If sulfa binds at a site other than the active site, is this a competitive or noncompetitive inhibitor? (covalent, ionic 2) It is irreversible, so the bond formed between the enzyme and antibiotic is likely. or hydrogen bond)? 3) Is the portion of sulfa shown polar or nonpolar? 4) What is a possible amino acid at the binding site of sulfa?
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter23: Enzymes
Section: Chapter Questions
Problem 23.25P: 5 Which of the following is a correct statement describing the induced-fit model of enzyme action?...
Related questions
Question
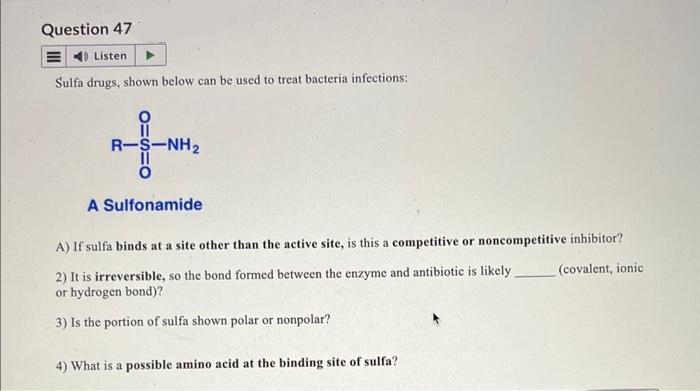
Transcribed Image Text:Question 47
Listen
Sulfa drugs, shown below can be used to treat bacteria infections:
R-S-NH₂
A Sulfonamide
A) If sulfa binds at a site other than the active site, is this a competitive or noncompetitive inhibitor?
2) It is irreversible, so the bond formed between the enzyme and antibiotic is likely.
or hydrogen bond)?
(covalent, ionic
3) Is the portion of sulfa shown polar or nonpolar?
4) What is a possible amino acid at the binding site of sulfa?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning



General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning