Chapter10: Potentiometry And Redox Titrations
Section: Chapter Questions
Problem 1P
Related questions
Question
#47 & 48
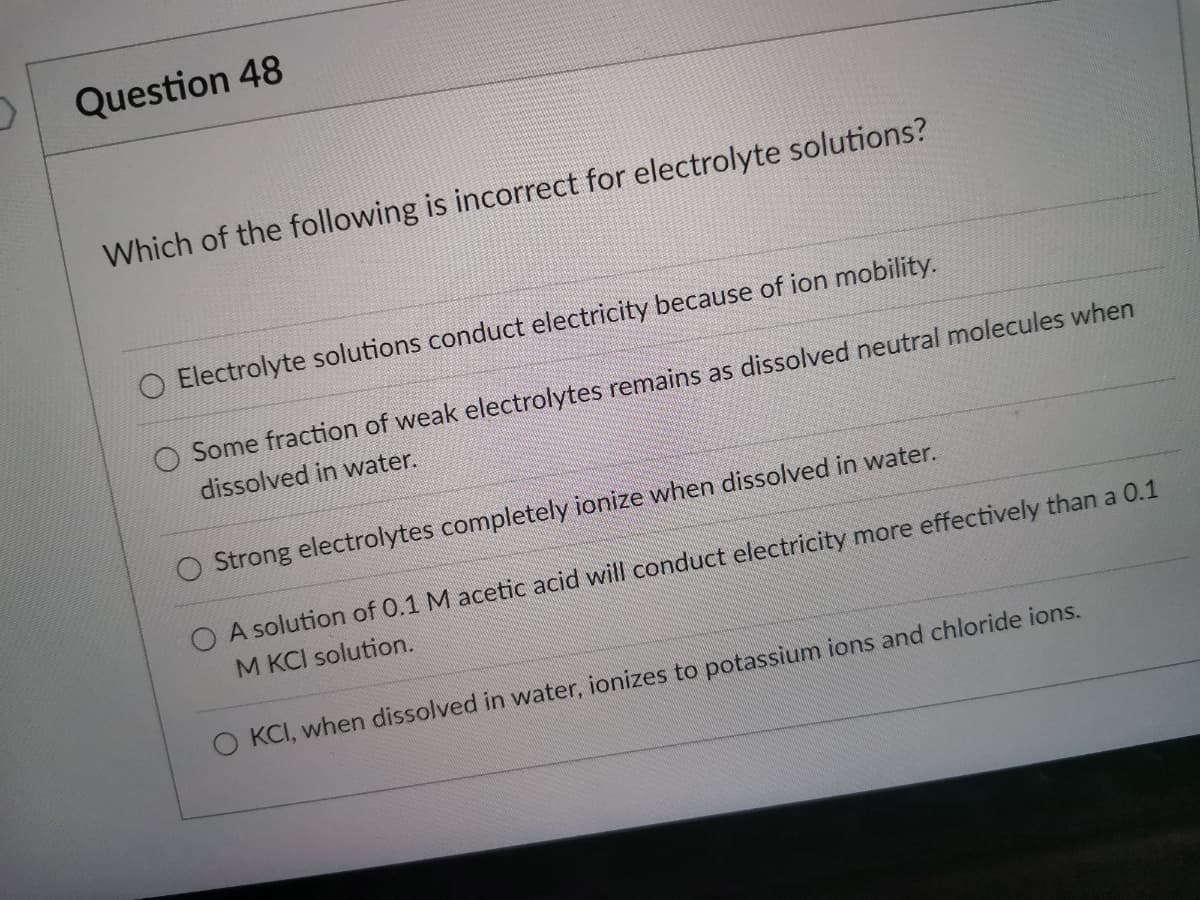
Transcribed Image Text:Question 48
Which of the following is incorrect for electrolyte solutions?
Electrolyte solutions conduct electricity because of ion mobility.
Some fraction of weak electrolytes remains as dissolved neutral molecules when
dissolved in water.
O Strong electrolytes completely ionize when dissolved in water.
O A solution of 0.1 M acetic acid will conduct electricity more effectively than a 0.1
M KCI solution.
O KCI, when dissolved in water, ionizes to potassium ions and chloride ions.

Transcribed Image Text:Question 47
What is the ionic strength of a 0.100 M solution of Na2SO4 with 0.2 M KI?
Ou= 0.250
OH= 0.200
OH= 0.500
OH= 0.350
%3D
Question 48
lutions?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

