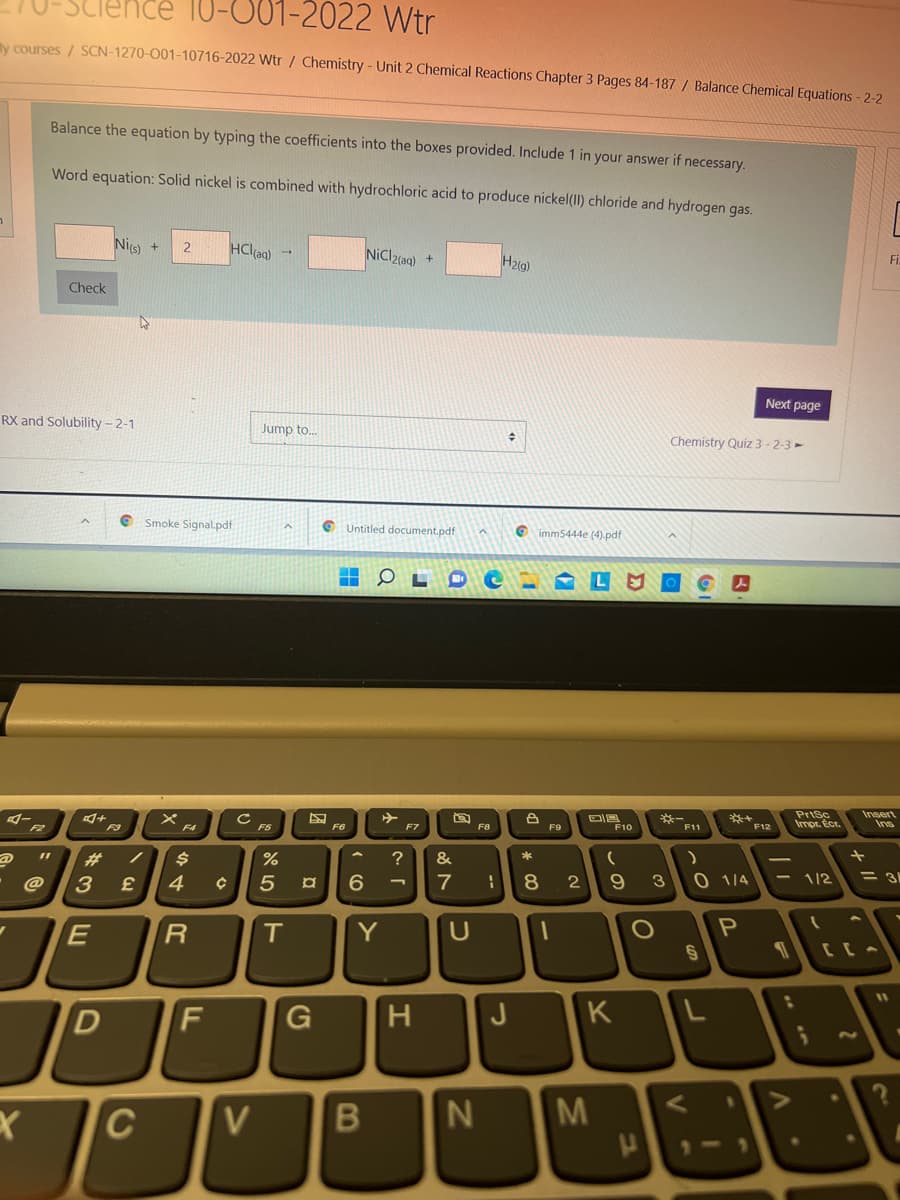
1-10716-2022 Wtr / Chemistry- Unit 2 Chemical Reactions Chapter 3 Pages 84-187 / Balance Chemical Equations -2-2 Balance the equation by typing the coefficients into the boxes provided. Include 1 in your answer if necessary. Word equation: Solid nickel is combined with hydrochloric acid to produce nickel(II) chloride and hydrogen gas. Fi HCla) NiCl2(ag) Hzig) Nis) Check
1-10716-2022 Wtr / Chemistry- Unit 2 Chemical Reactions Chapter 3 Pages 84-187 / Balance Chemical Equations -2-2 Balance the equation by typing the coefficients into the boxes provided. Include 1 in your answer if necessary. Word equation: Solid nickel is combined with hydrochloric acid to produce nickel(II) chloride and hydrogen gas. Fi HCla) NiCl2(ag) Hzig) Nis) Check
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter6: Chemical Reactions: An Introduction
Section: Chapter Questions
Problem 20QAP: Many over-the-counter antacid tablets are now formulated using calcium carbonate as the active...
Related questions
Question
Transcribed Image Text:ice 10-001-2022 Wtr
y courses / SCN-1270-001-10716-2022 Wtr / Chemistry - Unit 2 Chemical Reactions Chapter 3 Pages 84-187 / Balance Chemical Equations - 2-2
Balance the equation by typing the coefficients into the boxes provided. Include 1 in your answer if necessary.
Word equation: Solid nickel is combined with hydrochloric acid to produce nickel(II) chloride and hydrogen gas.
Nis)
HCl(ag) -
NiCl2(aq) +
H2(g)
Fi.
Check
Next page
RX and Solubility – 2-1
Jump to.
Chemistry Quiz 3 - 2-3 -
O Smoke Signal.pdf
Untitled document.pdf
O imm5444e (4).pdf
一
F11
Prisc
Impr. Ecr.
Insert
Ins
F2
F3
F4
F5
F6
F7
F8
F9
F10
F12
%23
&
3
4
7
3
O 1/4
- 112
ニ 3
@
2
R
Y
O
%3D
F
H
K
2
C
B
1-3
3D
* 00
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning