Question 5 temperature and pressure whereby CO2, H2O, and N2 react to form C9H28O2N and O2. In the reaction, the student had 14.57 moles of CO2, 128.75 grams of H2O, and 59.7 grams of N2, and generated only 79.27 grams of C9H28O2N. Please determine the limiting reagent, the theoretical yield, and the percent yield. A student performed a reaction at high
Question 5 temperature and pressure whereby CO2, H2O, and N2 react to form C9H28O2N and O2. In the reaction, the student had 14.57 moles of CO2, 128.75 grams of H2O, and 59.7 grams of N2, and generated only 79.27 grams of C9H28O2N. Please determine the limiting reagent, the theoretical yield, and the percent yield. A student performed a reaction at high
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.32E
Related questions
Question
help
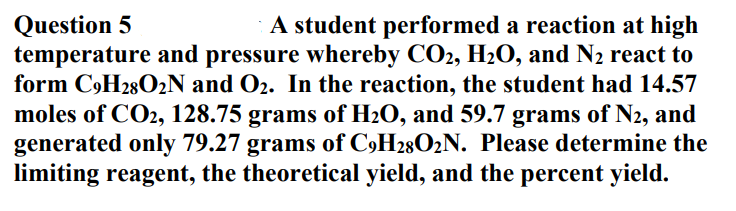
Transcribed Image Text:Question 5
temperature and pressure whereby CO2, H2O, and N2 react to
form C,H28O2N and O2. In the reaction, the student had 14.57
moles of CO2, 128.75 grams of H2O, and 59.7 grams of N2, and
generated only 79.27 grams of C9H28O2N. Please determine the
limiting reagent, the theoretical yield, and the percent yield.
A student performed a reaction at high
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning