QUESTION 50.2 g of KCIO3 (M-122.6 g/mol) is dissolved in enough water to prepare 875 mL of solution. What is the mass % of the solution if its density is 1.06 g/mL pMac
QUESTION 50.2 g of KCIO3 (M-122.6 g/mol) is dissolved in enough water to prepare 875 mL of solution. What is the mass % of the solution if its density is 1.06 g/mL pMac
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 23QAP
Related questions
Question

Transcribed Image Text:QUESTION
50.2 g of KCIO03 (M-122.6 g/mol) is dissolved in enough water to prepare 875 mL of solution.
What is the mass % of the solution if its density is 1.06 g/mL

Transcribed Image Text:QUESTION 7
What effect will increasing the pressure have on the system?
PO10(s)=P1(s) +5 02(g).
A) The reaction will shift to the right in the direction of products.
B) The reaction will shift to the left in the direction of reactants.
C) The equilibrium constant will increase.
D) The equilibrium constant will decrease.
E) No effect will be observed.
Expert Solution
Question 8
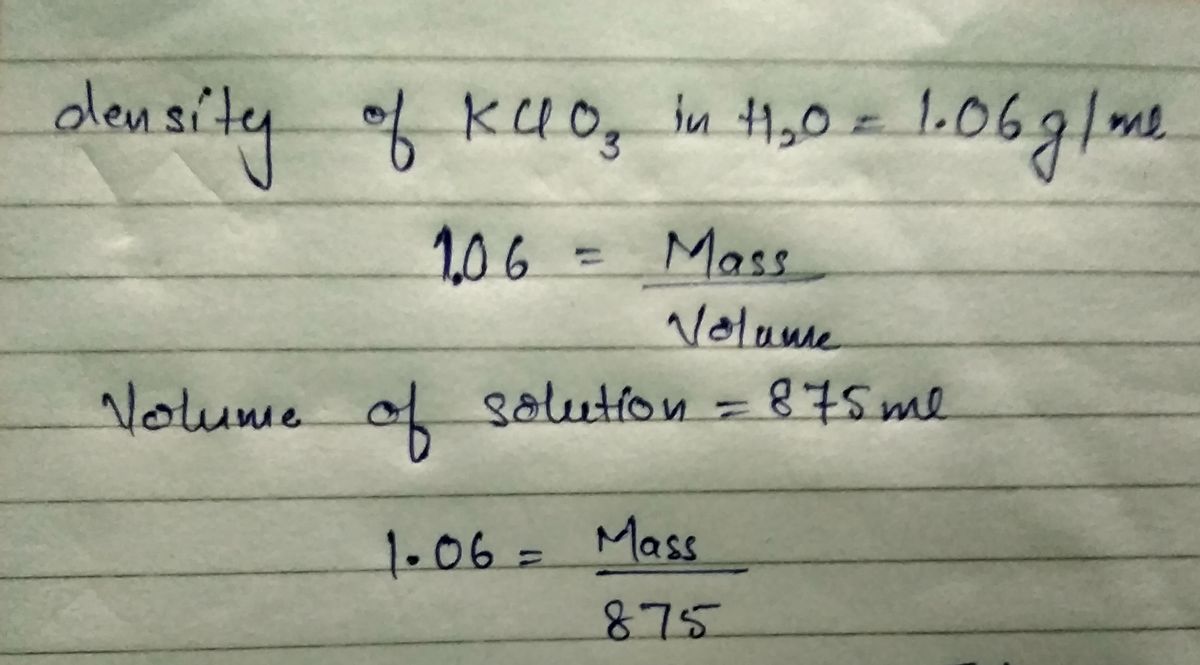
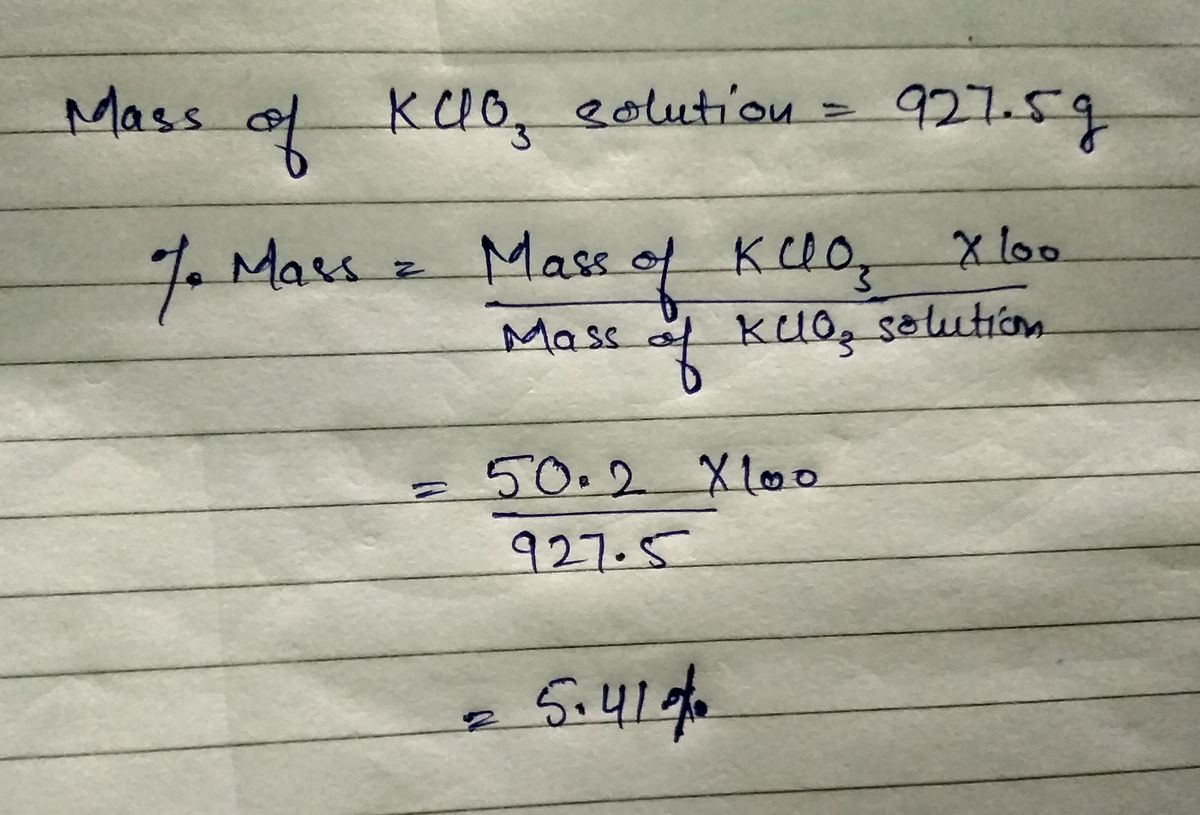
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning