Reports e-Services Y Academic Departments ETC - CIMS - Al Jula ion 27 Answer the following questions by using mole concept. et red (a) Calculate the molarity of a 155 g Na2SO4 in 770 ml d out of solution. [Atomic mass: Na=23 g/mol, S= 32 g/mol, O=16 g/mol, C=12 g/mol, H= 1 g/mol] in (a1)no of moles of Na2SO4 (a2) molarity of solution (b) 34 g C6H1206 is dissolved in 450 g of water. Find out the molality of the solution. (b1) no of moles of C6H1206 (b2) molality of solution
Reports e-Services Y Academic Departments ETC - CIMS - Al Jula ion 27 Answer the following questions by using mole concept. et red (a) Calculate the molarity of a 155 g Na2SO4 in 770 ml d out of solution. [Atomic mass: Na=23 g/mol, S= 32 g/mol, O=16 g/mol, C=12 g/mol, H= 1 g/mol] in (a1)no of moles of Na2SO4 (a2) molarity of solution (b) 34 g C6H1206 is dissolved in 450 g of water. Find out the molality of the solution. (b1) no of moles of C6H1206 (b2) molality of solution
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 16QAP
Related questions
Question
![Reports
e-Services ▼
Academic Departments
ETC -
CIMS -
Al Juland
ion 27
Answer the following questions by using mole concept.
et
red
(a) Calculate the molarity of a 155 g Na2SO4 in 770 ml
d out of
solution.
[Atomic mass: Na=23 g/mol, S= 32 g/mol, O=16 g/mol,
C=12 g/mol, H= 1 g/mol]
in
(a1)no of moles of Na2SO4
(a2) molarity of solution
(b) 34 g C6H12O6 is dissolved in 450 g of water. Find
out the molality of the solution.
(b1) no of moles of C6H1206
(b2) molality of solution
(c) How much volume of 1.5 M NaOH solution can be
made by diluting 56 ml of 4 M NaOH.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F3f2ca1b4-3928-4105-9cfb-cc03cd8f6402%2F51abc0b0-b7e7-4315-bbdc-bbb81ee9b503%2Fhrmz4j_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Reports
e-Services ▼
Academic Departments
ETC -
CIMS -
Al Juland
ion 27
Answer the following questions by using mole concept.
et
red
(a) Calculate the molarity of a 155 g Na2SO4 in 770 ml
d out of
solution.
[Atomic mass: Na=23 g/mol, S= 32 g/mol, O=16 g/mol,
C=12 g/mol, H= 1 g/mol]
in
(a1)no of moles of Na2SO4
(a2) molarity of solution
(b) 34 g C6H12O6 is dissolved in 450 g of water. Find
out the molality of the solution.
(b1) no of moles of C6H1206
(b2) molality of solution
(c) How much volume of 1.5 M NaOH solution can be
made by diluting 56 ml of 4 M NaOH.
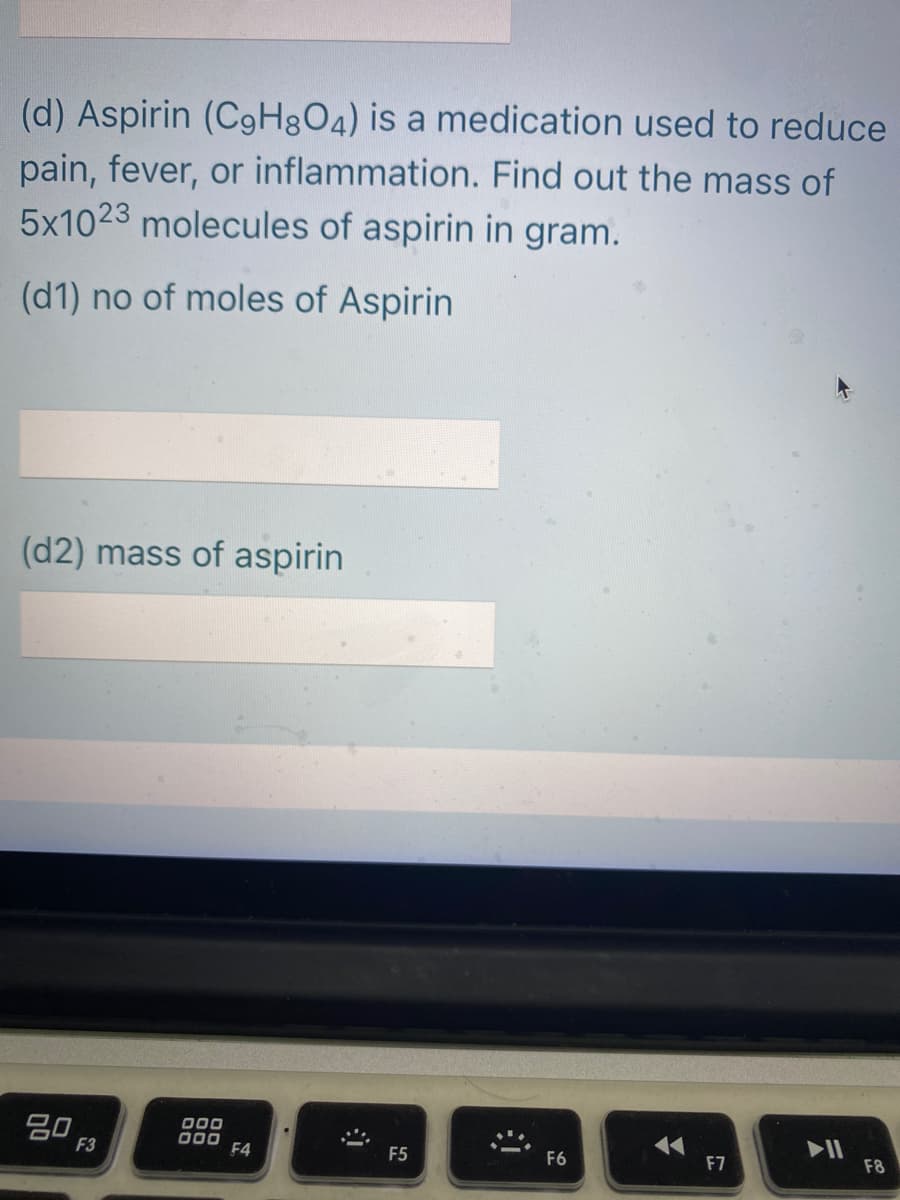
Transcribed Image Text:(d) Aspirin (C9H3O4) is a medication used to reduce
pain, fever, or inflammation. Find out the mass of
5x1023 molecules of aspirin in gram.
(d1) no of moles of Aspirin
(d2) mass of aspirin
20
F3
000
p00 F4
17
F7
F5
F6
F8
Expert Solution
Step 1
 Molarity is defined as number of moles of solute present in the given solution in mL.
Molarity is defined as number of moles of solute present in the given solution in mL.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning