QUESTION 6 Please answer the questions below completely. Show ALL work for credit. Give all answers in 3 significant figures and scientific notation. Please calculate molecular weight (mass) to two numbers after the decimal. You can use the f. button to build you equations. Magnesium citrate, Mg3(C6H507)2, belongs to a class of laxatives called hyperosmotics, which cause rapid emptying of the bowel. When a concentrated solution of magnesium citrate is consumed, it passes through the intestines, drawing water and promoting diarrhea, usually within 6 hours. The recommended dose for a 30 year old male is approximately 400 mg per day. 1. Determine how may moles of Magnesium citrate is in the recommended does. 2. Determine how may total moles of ions are in the recommended does.
QUESTION 6 Please answer the questions below completely. Show ALL work for credit. Give all answers in 3 significant figures and scientific notation. Please calculate molecular weight (mass) to two numbers after the decimal. You can use the f. button to build you equations. Magnesium citrate, Mg3(C6H507)2, belongs to a class of laxatives called hyperosmotics, which cause rapid emptying of the bowel. When a concentrated solution of magnesium citrate is consumed, it passes through the intestines, drawing water and promoting diarrhea, usually within 6 hours. The recommended dose for a 30 year old male is approximately 400 mg per day. 1. Determine how may moles of Magnesium citrate is in the recommended does. 2. Determine how may total moles of ions are in the recommended does.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
ChapterL: Let's Review
SectionL.3: Mathematics Of Chemistry
Problem 4RC
Related questions
Question
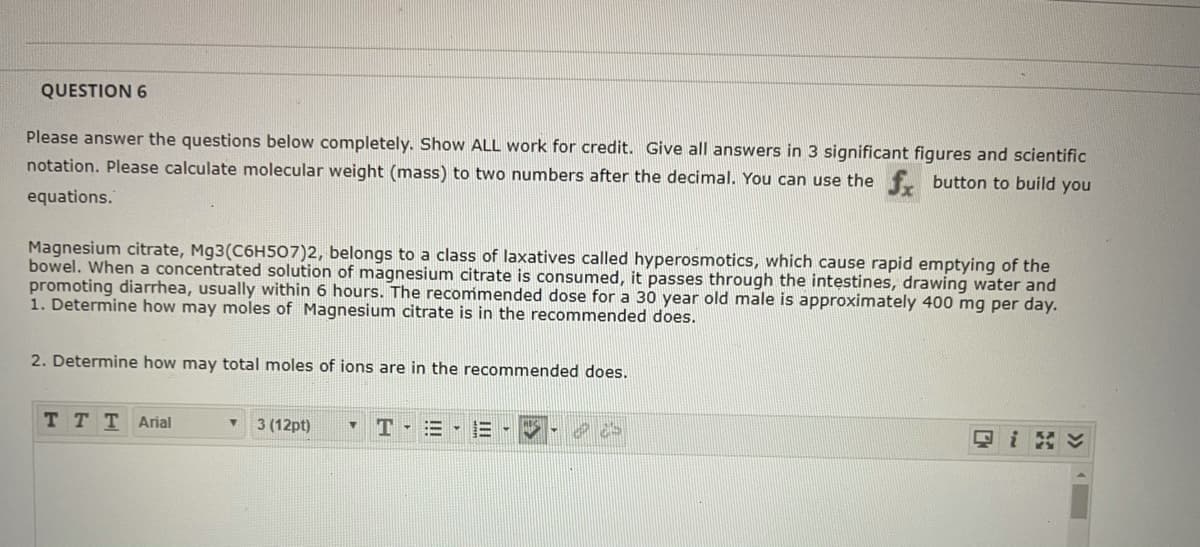
Transcribed Image Text:QUESTION 6
Please answer the questions below completely. Show ALL work for credit. Give all answers in 3 significant figures and scientific
notation. Please calculate molecular weight (mass) to two numbers after the decimal. You can use the f. button to build you
equations.
Magnesium citrate, Mg3(C6H507)2, belongs to a class of laxatives called hyperosmotics, which cause rapid emptying of the
bowel. When a concentrated solution of magnesium citrate is consumed, it passes through the intestines, drawing water and
promoting diarrhea, usually within 6 hours. The recommended dose for a 30 year old male is approximately 400 mg per day.
1. Determine how may moles of Magnesium citrate is in the recommended does.
2. Determine how may total moles of ions are in the recommended does.
T TTArial
3 (12pt)
T-E
回iaン
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning