QUESTION 9 Consider the dissolution of MnS in water (Kp = 3.0x10-14). MnS(s) + H20() Mn2 (aq) + HS-(aq) + OH-(aq) How is the solubility of manganese(Il) sulfide affected by the addition of aqueous potassium hydroxide to the system? Oa. The solubility will increase. Ob. The pK, of H,S is needed before a reliable prediction can be made. Oc. The amount of KOH added must be known before its effect can be predicted Od The solubility will be unchanged. Oe. The solubility will decrease.
QUESTION 9 Consider the dissolution of MnS in water (Kp = 3.0x10-14). MnS(s) + H20() Mn2 (aq) + HS-(aq) + OH-(aq) How is the solubility of manganese(Il) sulfide affected by the addition of aqueous potassium hydroxide to the system? Oa. The solubility will increase. Ob. The pK, of H,S is needed before a reliable prediction can be made. Oc. The amount of KOH added must be known before its effect can be predicted Od The solubility will be unchanged. Oe. The solubility will decrease.
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 6P
Related questions
Concept explainers
Question
100%
Pls help ASAP
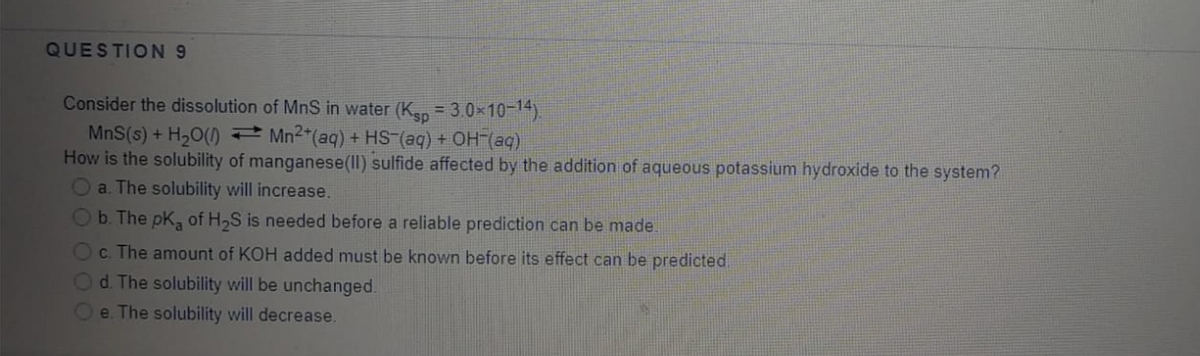
Transcribed Image Text:QUESTION9
Consider the dissolution of MnS in water (Kan = 3.0x10-14)
MnS(s) + H20() Mn2"(aq) + HS (aq) + OH (aq)
How is the solubility of manganese(II) sulfide affected by the addition of aqueous potassium hydroxide to the system?
a. The solubility will increase.
b. The pK, of H,S is needed before a reliable prediction can be made.
c. The amount of KOH added must be known before its effect can be predicted.
Od The solubility will be unchanged.
e. The solubility will decrease.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

