Question 9 If reaction Pathway A is carried out at high temperatures, a trisubstituted product forms. Which of the following compounds is the possible trisubstituted derivative? O2N- -Br O2N- -Br -Br Br A. O,N B Br NO2 NO2 O,N- -Br ON- -NO2 Question 10 Which of the following is a correct statement regarding electrophilic aromatic substitution? A. The carbocation intermediate will lose a proton to regain aromaticity, usually from a position other than the site of electrophilic attack. B. Formation of the carbocation intermediate has a high activation barrier due to loss of aromaticity. C. The carbocation intermediate has several resonance structures and is negatively charged. D. Reformation of the aromatic ring has a low activation barrier and therefore occurs slowly. E. Many suitable electrophiles are unreactive and can be stored for long periods of time prior to use.
Question 9 If reaction Pathway A is carried out at high temperatures, a trisubstituted product forms. Which of the following compounds is the possible trisubstituted derivative? O2N- -Br O2N- -Br -Br Br A. O,N B Br NO2 NO2 O,N- -Br ON- -NO2 Question 10 Which of the following is a correct statement regarding electrophilic aromatic substitution? A. The carbocation intermediate will lose a proton to regain aromaticity, usually from a position other than the site of electrophilic attack. B. Formation of the carbocation intermediate has a high activation barrier due to loss of aromaticity. C. The carbocation intermediate has several resonance structures and is negatively charged. D. Reformation of the aromatic ring has a low activation barrier and therefore occurs slowly. E. Many suitable electrophiles are unreactive and can be stored for long periods of time prior to use.
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter2: Measurements And Calculations
Section: Chapter Questions
Problem 104AP: Convert 45 mi/h to m/s, showing how the units cancel appropriately.
Related questions
Question
9 and 10
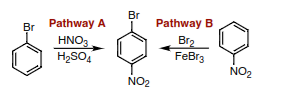
Transcribed Image Text:Br
Pathway A
Pathway B
Br
HNO3
H2SO,
Br2
FeBr3
NO2
NO2
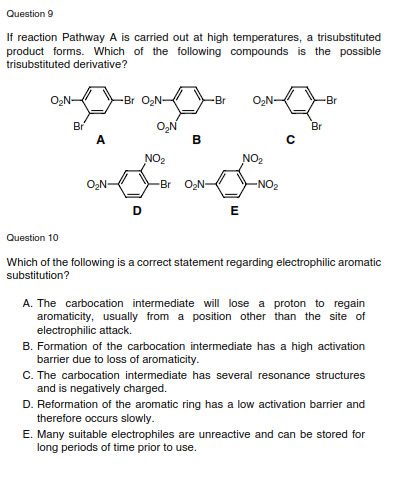
Transcribed Image Text:Question 9
If reaction Pathway A is carried out at high temperatures, a trisubstituted
product forms. Which of the following compounds is the possible
trisubstituted derivative?
-Br O,N-
-Br
-Br
O,N
B
Br
Br
A.
NO2
NO2
O2N-
-Br ON-
-NO2
E
Question 10
Which of the following is a correct statement regarding electrophilic aromatic
substitution?
A. The carbocation intermediate will lose a proton to regain
aromaticity, usually from a position other than the site of
electrophilic attack.
B. Formation of the carbocation intermediate has a high activation
barrier due to loss of aromaticity.
C. The carbocation intermediate has several resonance structures
and is negatively charged.
D. Reformation of the aromatic ring has a low activation barrier and
therefore occurs slowly.
E. Many suitable electrophiles are unreactive and can be stored for
long periods of time prior to use.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

