Question: How does temperature affect the pressure of a gas when volume is constant? 1. Form hypothesis: If the volume of a gas is held constant, how do you think the pressure will change as temperature increases? 2. Collect data: Select the TABLE tab. Record the pressure when T = 100 K, 200 K, and so forth up to 500 K. (Note: The volume will remain constant at 1.02 m3.) Pressure Temperature Pressure Temperature 100 K 200 K 300 K 400 K 500 K 3. Analyze: Divide the pressure by the temperature to fill in the last column of the table. Since 1 N/m? is equal to 1 pascal (Pa), write the units of the ratio as Pa/K. A. When the volume is held constant, how does the pressure change as temperature increases? B. What do you notice about the ratio of pressure to temperature, when volume is constant? Gay-Lussac's law states that, at constant volume, the ratio of pressure to temperature is constant. As temperature increases, pressure increases as well.
Question: How does temperature affect the pressure of a gas when volume is constant? 1. Form hypothesis: If the volume of a gas is held constant, how do you think the pressure will change as temperature increases? 2. Collect data: Select the TABLE tab. Record the pressure when T = 100 K, 200 K, and so forth up to 500 K. (Note: The volume will remain constant at 1.02 m3.) Pressure Temperature Pressure Temperature 100 K 200 K 300 K 400 K 500 K 3. Analyze: Divide the pressure by the temperature to fill in the last column of the table. Since 1 N/m? is equal to 1 pascal (Pa), write the units of the ratio as Pa/K. A. When the volume is held constant, how does the pressure change as temperature increases? B. What do you notice about the ratio of pressure to temperature, when volume is constant? Gay-Lussac's law states that, at constant volume, the ratio of pressure to temperature is constant. As temperature increases, pressure increases as well.
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU3: Weather: Phase Changes And Behaviour Of Gases
SectionU3.10: Feeling Under Pressure: Boyle's Law
Problem 7E
Related questions
Question
100%
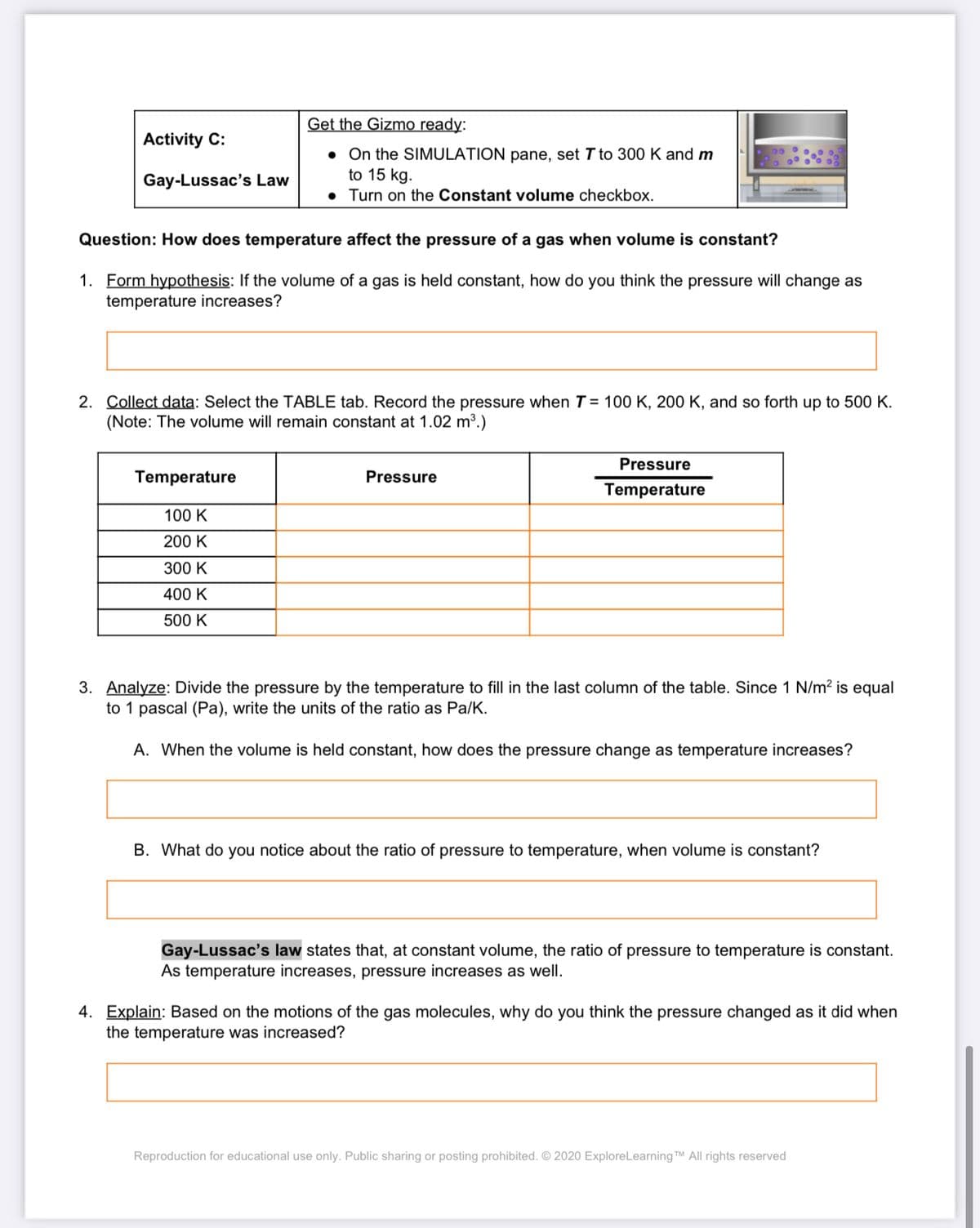
Transcribed Image Text:Get the Gizmo ready:
Activity C:
• On the SIMULATION pane, set T to 300 K and m
to 15 kg.
• Turn on the Constant volume checkbox.
Gay-Lussac's Law
Question: How does temperature affect the pressure of a gas when volume is constant?
1. Form hypothesis: If the volume of a gas is held constant, how do you think the pressure will change as
temperature increases?
2. Collect data: Select the TABLE tab. Record the pressure when T = 100 K, 200 K, and so forth up to 500 K.
(Note: The volume will remain constant at 1.02 m³.)
Pressure
Temperature
Pressure
Temperature
100 K
200 K
300 K
400 K
500 K
3. Analyze: Divide the pressure by the temperature to fill in the last column of the table. Since 1 N/m? is equal
to 1 pascal (Pa), write the units of the ratio as Pa/K.
A. When the volume is held constant, how does the pressure change as temperature increases?
B. What do you notice about the ratio of pressure to temperature, when volume is constant?
Gay-Lussac's law states that, at constant volume, the ratio of pressure to temperature is constant.
As temperature increases, pressure increases as well.
4. Explain: Based on the motions of the gas molecules, why do you think the pressure changed as it did when
the temperature was increased?
Reproduction for educational use only. Public sharing or posting prohibited. © 2020 ExploreLearning TM All rights reserved

Transcribed Image Text:6. Calculate: Compare the pressure and volume values in your data table.
A. How did doubling the temperature affect the gas volume?
B. How did tripling the temperature affect the gas volume?
C. How did quadrupling the temperature affect the gas volume?
7. Predict: Suppose the temperature was 50 K. What will be the volume of the gas?
8. Test: Test your prediction using the Gizmo. What is the volume of the gas?
Was your prediction correct?
9. Create a graph: On the GRAPH tab, select V vs. T. Set I to 50 K, and click Record to plot a point on the
graph. Plot a point every 50 degrees to create a graph showing the relationship between temperature and
volume.
When your graph is completed, click the camera icon to take a snapshot. Paste the image into the space
below, and label the graph "Volume vs. Temperature."
D. What is the shape of the graph?
E. How does this graph illustrate
Charles's law?
10. Apply: Based on what you learned, what would happen to a balloon placed in the freezer?
What would happen to a balloon placed in a warm oven? (Assume it doesn't pop.)
11. Think and discuss: Consider temperature, pressure, and volume. How does the mathematical relationship
in Boyle's law compare to that in Charles's law?
Reproduction for educational use only. Public sharing or posting prohibited. © 2020 ExploreLearning TM All rights reserved
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning