Quinine is a natural product extracted from the bark of the cinchona tree, which is native to South America. Quinine is used as an antimalarial agent. When 2.35 g of quinine is dissolved in 25.0 g of cyclohexane, the freezing point of the solution is lowered by 6.04 "C. Look up the freezing point and K, constant for cyclohexane in the Colligative Constants table. Calculate the molar mass of quinine. 313 molar mass: g/mol
Quinine is a natural product extracted from the bark of the cinchona tree, which is native to South America. Quinine is used as an antimalarial agent. When 2.35 g of quinine is dissolved in 25.0 g of cyclohexane, the freezing point of the solution is lowered by 6.04 "C. Look up the freezing point and K, constant for cyclohexane in the Colligative Constants table. Calculate the molar mass of quinine. 313 molar mass: g/mol
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 7E
Related questions
Question
100%
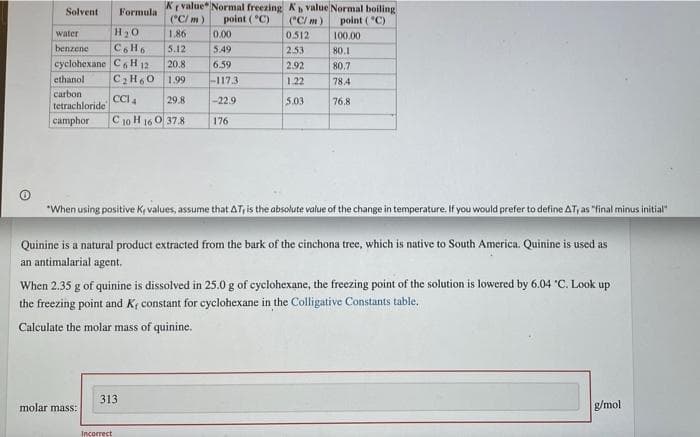
Transcribed Image Text:Solvent Formula
Kr value Normal freezing
(°C/m)
Kb value Normal boiling
(°C/m) point (°C)
point (°C)
water
H₂O
1.86
0.00
0.512
100.00
5.12
5.49
2.53
80.1
benzene C6H6
cyclohexane C6H12 20.8
6.59
2.92
80.7
ethanol C₂H60 1.99
-117.3:
1.22
78.4
carbon
CCI 4
29.8
-22.9
5.03
76,8
tetrachloride
camphor C10 H 16 0 37.8
176
"When using positive K, values, assume that AT, is the absolute value of the change in temperature. If you would prefer to define AT, as "final minus initial"
Quinine is a natural product extracted from the bark of the cinchona tree, which is native to South America. Quinine is used as
an antimalarial agent.
When 2.35 g of quinine is dissolved in 25.0 g of cyclohexane, the freezing point of the solution is lowered by 6.04 "C. Look up
the freezing point and K, constant for cyclohexane in the Colligative Constants table.
Calculate the molar mass of quinine.
313
g/mol
molar mass:
e
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT