(a) Consider the polymerization of isoprene (see below for the formula): Draw the chemical structure of at least five different polymers and/or polymer isomers that may be formed from this monomer CH₂ H₂C C=CH₂ (b) Sketch the different types of copolymers that can be obtained from the copolymerization of two monomers A and B. If A can form semicrystalline homopolymer while B forms an amorphous one, which copolymer types would be crystallizable?
(a) Consider the polymerization of isoprene (see below for the formula): Draw the chemical structure of at least five different polymers and/or polymer isomers that may be formed from this monomer CH₂ H₂C C=CH₂ (b) Sketch the different types of copolymers that can be obtained from the copolymerization of two monomers A and B. If A can form semicrystalline homopolymer while B forms an amorphous one, which copolymer types would be crystallizable?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter23: Organic Polymers, Natural And Synthetic
Section: Chapter Questions
Problem 51QAP
Related questions
Question
Please solve this
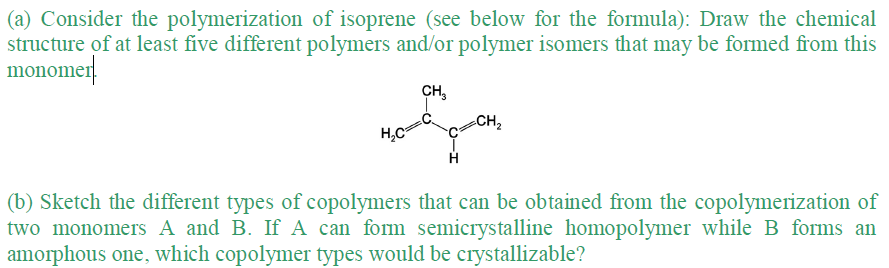
Transcribed Image Text:(a) Consider the polymerization of isoprene (see below for the formula): Draw the chemical
structure of at least five different polymers and/or polymer isomers that may be formed from this
monomer
CH₂
CH ₂
me
H₂C²
H
(b) Sketch the different types of copolymers that can be obtained from the copolymerization of
two monomers A and B. If A can form semicrystalline homopolymer while B forms an
amorphous one, which copolymer types would be crystallizable?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning