Re Kinetics Assignment 1) A chemical reaction proceeds with the proposed reaction mechanism below; +175 SCH4U0 E (kJ/mol) 75AH Step Ne) + Oze) → N,0 +Ow. 25- AH 1 +50 N20 +Oze) → NOe) +NO) -150+AH +25 NO +Ow + N0) a) Draw the potential energy diagram for this reaction below. 300 250 P. E. (k 200 J/ 150 mo 1) 100 Ne +0 2(g) 2(g) 50 b) What is the overall reaction? N2(g) + 20xg -> c) Which step is the rate determining step? Explain why. Step 2 because it has the highest activation energy. d) What would be the rate law for this reaction? e) Explain what would happen to the rate if the concentration of N doubled. f) Use a dotted line to show what would happen if a catalyst was added to the reaction. g) Identify the spot on the graph that represents the transition state for reaction 2. h) Identify the intermediates present in this reaction. (Intermediates are produced and consumed in the reaction mechanisms)
Re Kinetics Assignment 1) A chemical reaction proceeds with the proposed reaction mechanism below; +175 SCH4U0 E (kJ/mol) 75AH Step Ne) + Oze) → N,0 +Ow. 25- AH 1 +50 N20 +Oze) → NOe) +NO) -150+AH +25 NO +Ow + N0) a) Draw the potential energy diagram for this reaction below. 300 250 P. E. (k 200 J/ 150 mo 1) 100 Ne +0 2(g) 2(g) 50 b) What is the overall reaction? N2(g) + 20xg -> c) Which step is the rate determining step? Explain why. Step 2 because it has the highest activation energy. d) What would be the rate law for this reaction? e) Explain what would happen to the rate if the concentration of N doubled. f) Use a dotted line to show what would happen if a catalyst was added to the reaction. g) Identify the spot on the graph that represents the transition state for reaction 2. h) Identify the intermediates present in this reaction. (Intermediates are produced and consumed in the reaction mechanisms)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section11.1: Reaction Rate
Problem 11.2CE: Instantaneous rates for the reaction of hydroxide ion with Cv+ can be determined from the slope of...
Related questions
Question
100%
The other questions we’re answered already I just need e, f , g and h please.

Transcribed Image Text:amples:
Unimeleculo
gras a
Teact
Eimaan
e
- Rea
1) A chemical reaction proceeds with the proposed reaction mechanism below;
Step
Kinetics Assignment
SCH4U0
E (kJ/mol)
75AH
+175
25-AH
+50
N2e) + Oze) → N,O« +O«).
1
+25
-150+0H
N2O +Oz«) → NOe) +NO)
NOw +O«) → N0ze)
a) Draw the potential energy diagram for this reaction below.
300
250
P.
E.
200
(k
J/
150
mo
1)
100
+0218)
50
b) What is the overall reaction?
N2 9) + 2 Ozcg
->
c) Which step is the rate determining step? Explain why.
Step 2 because it has the highest activation energy.
d) What would be the rate law for this reaction?
K= [
e) Explain what would happen to the rate if the concentration of Ne
doubled.
f) Use a dotted line to show what would happen if a catalyst was added to the reaction.
g) Identify the spot on the graph that represents the transition state for reaction 2.
h) Identify the intermediates present in this reaction. (Intermediates are produced and
consumed in the reaction mechanisms)
Transcribed Image Text:11:17
1 Gmail
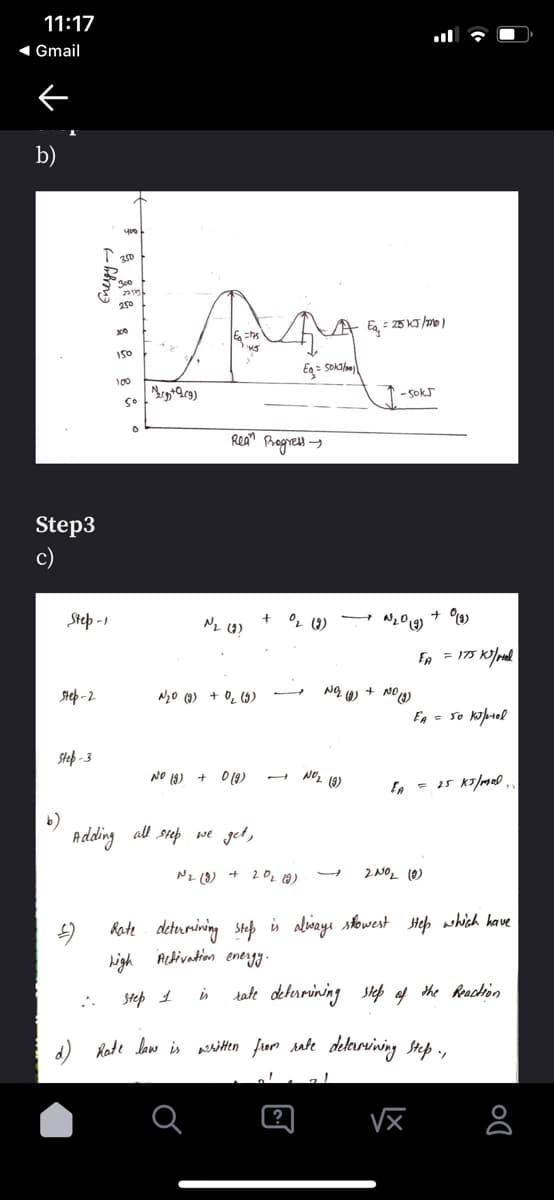
b)
T 20
300
250
100
So
Rean Brogress -
Step3
c)
Step -1
- N2019)
Ep = 175 K/rel
Step -2
NgO (9) + O_ (5)
En = so kouol
Step - 3
NO 19) + D(9)
b)
Adding al siep we get,
Nz (8) + 20L (0)
2NOL (0)
Rate deturiving Step is alinayı sowest Hep which have
high ALbivation energy.
Step 1
is
tate deterrining step of the Reaction
a) Rode law is asitten fiom sate deleırvining step .,
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
