Reaction A, an E2 reaction with a strong, small, base: KOH, Br 1-propanol Reaction B, an E2 reaction with a strong, bulky base: KOC(CH3)3 HOC(CH3)3 Br and/or and/or
Reaction A, an E2 reaction with a strong, small, base: KOH, Br 1-propanol Reaction B, an E2 reaction with a strong, bulky base: KOC(CH3)3 HOC(CH3)3 Br and/or and/or
Chapter3: Mechanisms
Section: Chapter Questions
Problem 104EQ
Related questions
Question
Which
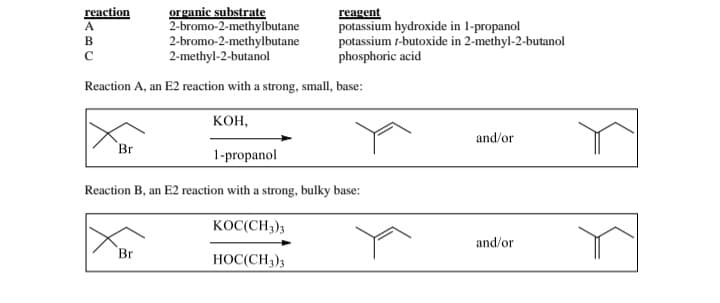
Transcribed Image Text:organic substrate
2-bromo-2-methylbutane
2-bromo-2-methylbutane
2-methyl-2-butanol
Reaction A, an E2 reaction with a strong, small, base:
KOH,
1-propanol
Reaction B, an E2 reaction with a strong, bulky base:
KOC(CH3)3
HOC(CH3)3
reaction
BABC
с
Br
Br
reagent
potassium hydroxide in 1-propanol
potassium 1-butoxide in 2-methyl-2-butanol
phosphoric acid
and/or
and/or
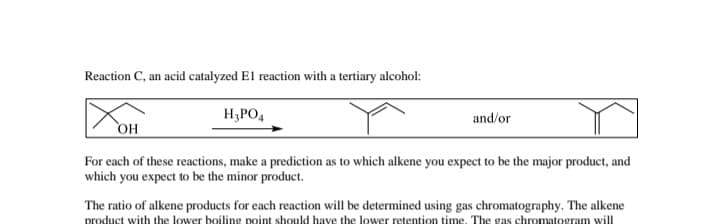
Transcribed Image Text:Reaction C, an acid catalyzed El reaction with a tertiary alcohol:
H3PO4
and/or
OH
For each of these reactions, make a prediction as to which alkene you expect to be the major product, and
which you expect to be the minor product.
The ratio of alkene products for each reaction will be determined using gas chromatography. The alkene
product with the lower boiling point should have the lower retention time. The gas chromatogram will
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

