Reaction Yield (2 parts) Organotin compounds play a significant role in diverse industrial applications. They have been used as plastic stabilizers and as pesticides or fungicides. One method used to prepare simple tetraalkylstannanes is the controlled direct reaction of liquid tin(IV) chloride with highly reactive trialkylaluminum compounds, such as liquid triethylaluminum (AI(C₂H5)3). 3SnCl4 + 4AI(C₂H5)33Sn(C₂H5)4 + 4AICI3 In one experiment, 0.280 L of SnCl4 (d = 2.226 g/mL) was treated with 0.484 L of triethylaluminum (AI(C₂H5)3); d = 0.835 g/mL). What is the theoretical yield in this experiment (mass of tetraethylstannane, Sn(C₂H5)4)? 1pts Submit Answer Incorrect. Tries 1/5 Previous Tries If 0.418 L of tetraethylstannane (d = 1.187 g/mL) were actually isolated in this experiment, what was the percent yield? % 1pts Submit Answer Tries 0/5 Copyright: Department of Chemistry, Simon Fraser University - SFU Chemistry 2000-2022 Post Discussion Send Feedback
Reaction Yield (2 parts) Organotin compounds play a significant role in diverse industrial applications. They have been used as plastic stabilizers and as pesticides or fungicides. One method used to prepare simple tetraalkylstannanes is the controlled direct reaction of liquid tin(IV) chloride with highly reactive trialkylaluminum compounds, such as liquid triethylaluminum (AI(C₂H5)3). 3SnCl4 + 4AI(C₂H5)33Sn(C₂H5)4 + 4AICI3 In one experiment, 0.280 L of SnCl4 (d = 2.226 g/mL) was treated with 0.484 L of triethylaluminum (AI(C₂H5)3); d = 0.835 g/mL). What is the theoretical yield in this experiment (mass of tetraethylstannane, Sn(C₂H5)4)? 1pts Submit Answer Incorrect. Tries 1/5 Previous Tries If 0.418 L of tetraethylstannane (d = 1.187 g/mL) were actually isolated in this experiment, what was the percent yield? % 1pts Submit Answer Tries 0/5 Copyright: Department of Chemistry, Simon Fraser University - SFU Chemistry 2000-2022 Post Discussion Send Feedback
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter5: Alkenes: Bonding, Nomenclature, And Properties
Section: Chapter Questions
Problem 5.31P
Related questions
Question
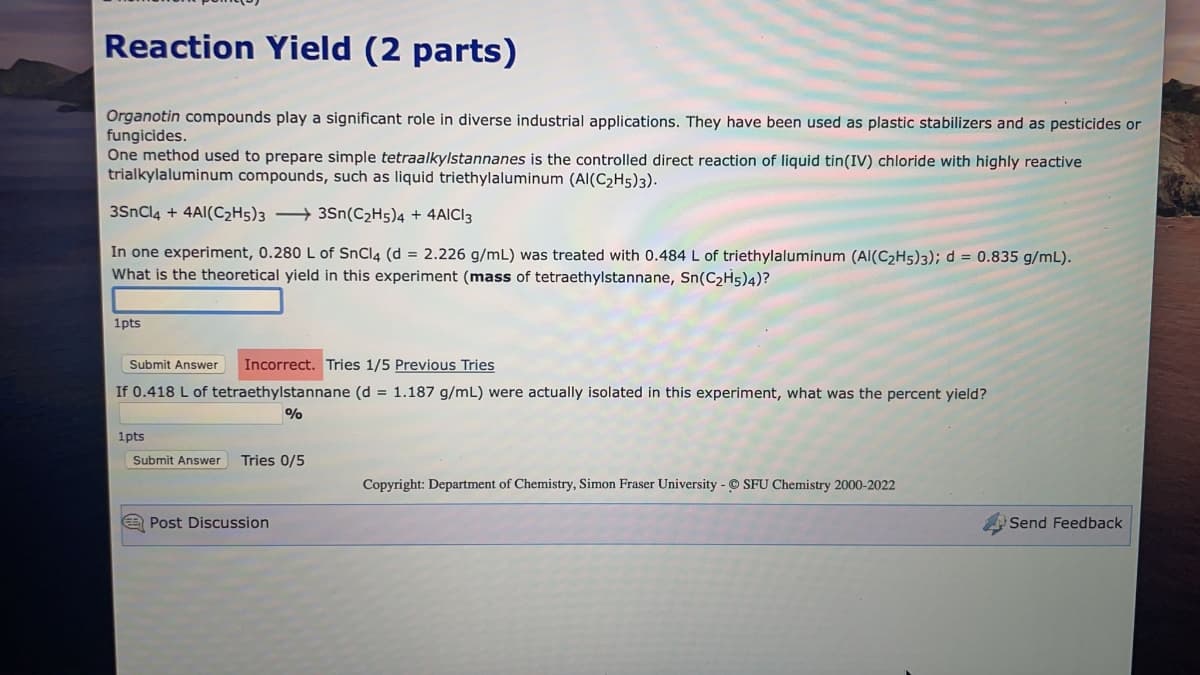
Transcribed Image Text:Reaction Yield (2 parts)
Organotin compounds play a significant role in diverse industrial applications. They have been used as plastic stabilizers and as pesticides or
fungicides.
One method used to prepare simple tetraalkylstannanes is the controlled direct reaction of liquid tin(IV) chloride with highly reactive
trialkylaluminum compounds, such as liquid triethylaluminum (AI(C₂H5)3).
3SnCl4 + 4AI(C2H5)33Sn(C₂H5)4 + 4AICI 3
In one experiment, 0.280 L of SnCl4 (d = 2.226 g/mL) was treated with 0.484 L of triethylaluminum (AI(C₂H5)3); d = 0.835 g/mL).
What is the theoretical yield in this experiment (mass of tetraethylstannane, Sn(C₂H5)4)?
1pts
Submit Answer Incorrect. Tries 1/5 Previous Tries
If 0.418 L of tetraethylstannane (d = 1.187 g/mL) were actually isolated in this experiment, what was the percent yield?
%
1pts
Submit Answer Tries 0/5
Copyright: Department of Chemistry, Simon Fraser University - SFU Chemistry 2000-2022
Post Discussion
Send Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning