REACTIONS 1. concentrated sulphuric acid + solid potassium chloride 2. concentrated sulphuric acid + solid potassium bromide 3. concentrated sulphuric acid + solid potassium iodide solid potassium chloride make a note of any changes Acidimetr y 1 1 1 presenc e of hydroge n halide gas Yes Yes Yes detectio presenc e of n of hydroge sulfur n sulfide dioxide gas gas Absent No Absent Yes Present Yes
REACTIONS 1. concentrated sulphuric acid + solid potassium chloride 2. concentrated sulphuric acid + solid potassium bromide 3. concentrated sulphuric acid + solid potassium iodide solid potassium chloride make a note of any changes Acidimetr y 1 1 1 presenc e of hydroge n halide gas Yes Yes Yes detectio presenc e of n of hydroge sulfur n sulfide dioxide gas gas Absent No Absent Yes Present Yes
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter20: Chemistry Of The Metals
Section: Chapter Questions
Problem 34QAP: The equilibrium constant for the reaction 2CrO42(aq)+2H+(aq)Cr2O72(aq)+H2O is 31014 . What must the...
Related questions
Question
Discuss the table
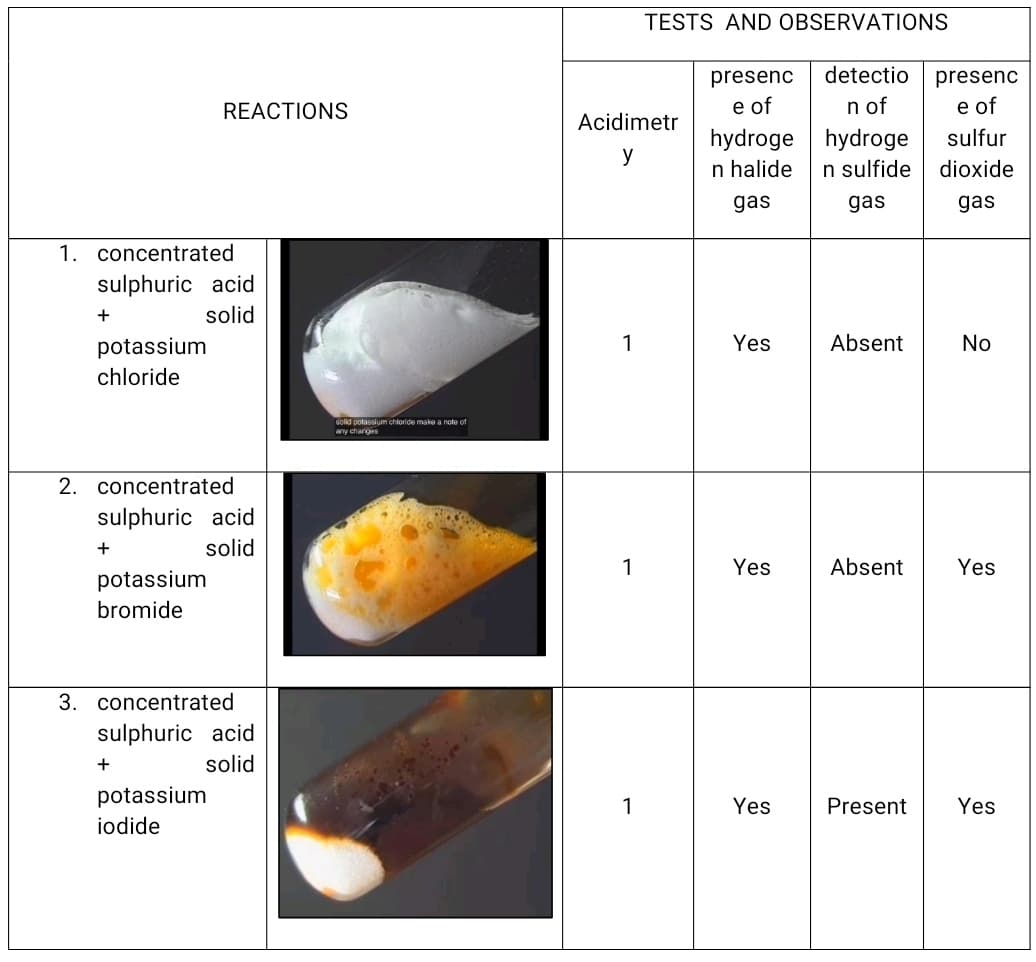
Transcribed Image Text:REACTIONS
1. concentrated
sulphuric acid
+
solid
potassium
chloride
2. concentrated
sulphuric acid
+
solid
potassium
bromide
3. concentrated
sulphuric acid
+
solid
potassium
iodide
solid potassium chloride make a note of
any changes
Acidimetr
y
1
1
TESTS AND OBSERVATIONS
1
presenc detectio presenc
e of
n of
e of
hydroge
hydroge
sulfur
n halide
n sulfide
dioxide
gas
gas
gas
Yes
Absent
No
Yes
Absent Yes
Yes
Present Yes
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning