Reagents Available a. CH2=CHCH,CI, AICI3 b. CН-Br2, ОН c. CH3CH2COCI, AICI3 d. H2, Pt e. NBS, CCI, f. K* tBuO g. CO, HCI, CUCI/AICI3 h. CH;CH2CH2COCI, AICI3 i. BrCH2CH2Br, он j. CH;CH2CI, AICI3 k. RCO3H I. H;O* CH3 Using conditions from the table, show how you would synthesize this compound from catechol (1,2-benzenediol).
Reagents Available a. CH2=CHCH,CI, AICI3 b. CН-Br2, ОН c. CH3CH2COCI, AICI3 d. H2, Pt e. NBS, CCI, f. K* tBuO g. CO, HCI, CUCI/AICI3 h. CH;CH2CH2COCI, AICI3 i. BrCH2CH2Br, он j. CH;CH2CI, AICI3 k. RCO3H I. H;O* CH3 Using conditions from the table, show how you would synthesize this compound from catechol (1,2-benzenediol).
Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.SE: Something Extra
Problem 40AP: From the data in Figure 4-12 and Table 4-1, estimate the percentages of molecules that have their...
Related questions
Question
Using conditions from the table, show how you would synthesize this compound from catechol (1,2-benzenediol).
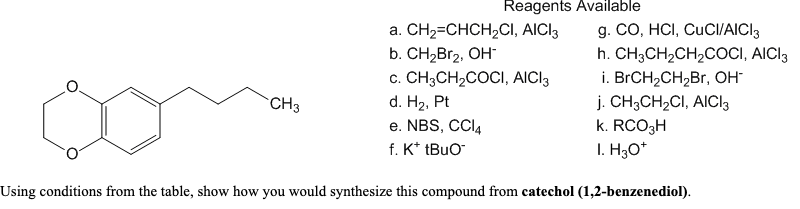
Transcribed Image Text:Reagents Available
a. CH2=CHCH,CI, AICI3
b. CН-Br2, ОН
c. CH3CH2COCI, AICI3
d. H2, Pt
e. NBS, CCI4
f. K* tBuO
g. CO, HCI, CUCI/AICI3
h. CH;CH2CH2COCI, AICI3
i. BrCH2CH2Br, он
j. CH;CH2CI, AICI3
k. RCO3H
I. H3O*
CH3
Using conditions from the table, show how you would synthesize this compound from catechol (1,2-benzenediol).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

