Recall from Section 2.10 that metal ions in hard water do not bind to the sulfate portion of detergents as strongly as they do to the carboxylate portion of soaps (alkyl sulfate = R-OSO3; carboxylate = R-CO,). Suggest a reason, based on intermolecular forces, for why this is so. 2.10 Soaps and Detergents Soaps and enzyme-free detergents belong to a class of compounds called surfactants. They remove dirt and oil from our hands, clothes, and dinnerware, all with no chemi- cal reaction occurring in the process (i.e., no covalent bonds are broken or formed). Instead, the cleansing ability of soaps and detergents depends entirely on intermo- lecular interactions. The molecules specifically responsible for a soap's cleansing properties are typically salts of fatty acids, which are ionic compounds of the form RCO, Na* or RCO, K*. In these compounds, R is a long hydrocarbon chain, and the fatty acid salts generally contain from 12 to 18 carbons. Examples include potassium oleate and sodium palmitate: CH3 CH3 Na H2ha Sodium palmitate C16H3102NA Potassium oleate C18H3302K When a soap dissolves in water, it does so as its individual ions: the metal cation (Na* or K*) and the carboxylate anion (RCO,). Of these two species, the carboxylate anion is the one that is directly responsible for the soap's cleansing properties, because it has vastly different characteristics at its two ends (Fig. 2-26). Specifically:
Recall from Section 2.10 that metal ions in hard water do not bind to the sulfate portion of detergents as strongly as they do to the carboxylate portion of soaps (alkyl sulfate = R-OSO3; carboxylate = R-CO,). Suggest a reason, based on intermolecular forces, for why this is so. 2.10 Soaps and Detergents Soaps and enzyme-free detergents belong to a class of compounds called surfactants. They remove dirt and oil from our hands, clothes, and dinnerware, all with no chemi- cal reaction occurring in the process (i.e., no covalent bonds are broken or formed). Instead, the cleansing ability of soaps and detergents depends entirely on intermo- lecular interactions. The molecules specifically responsible for a soap's cleansing properties are typically salts of fatty acids, which are ionic compounds of the form RCO, Na* or RCO, K*. In these compounds, R is a long hydrocarbon chain, and the fatty acid salts generally contain from 12 to 18 carbons. Examples include potassium oleate and sodium palmitate: CH3 CH3 Na H2ha Sodium palmitate C16H3102NA Potassium oleate C18H3302K When a soap dissolves in water, it does so as its individual ions: the metal cation (Na* or K*) and the carboxylate anion (RCO,). Of these two species, the carboxylate anion is the one that is directly responsible for the soap's cleansing properties, because it has vastly different characteristics at its two ends (Fig. 2-26). Specifically:
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:Recall from Section 2.10 that metal ions in hard water do not bind to the sulfate portion of detergents as strongly as they
do to the carboxylate portion of soaps (alkyl sulfate = R-OSO3; carboxylate = R-CO,). Suggest a reason, based on
intermolecular forces, for why this is so.
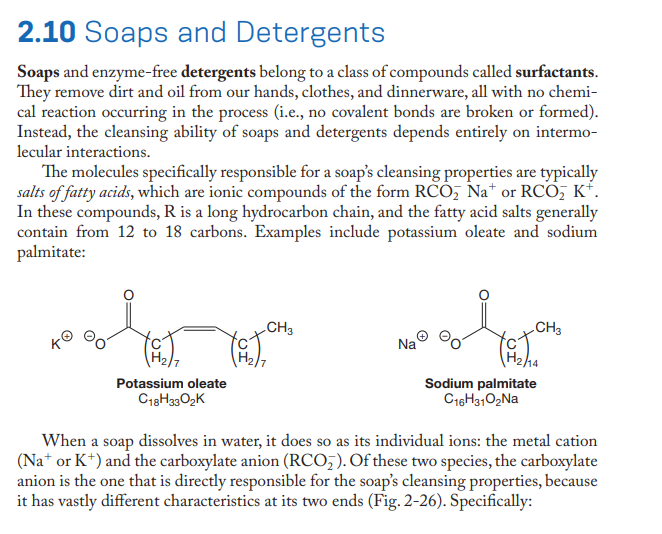
Transcribed Image Text:2.10 Soaps and Detergents
Soaps and enzyme-free detergents belong to a class of compounds called surfactants.
They remove dirt and oil from our hands, clothes, and dinnerware, all with no chemi-
cal reaction occurring in the process (i.e., no covalent bonds are broken or formed).
Instead, the cleansing ability of soaps and detergents depends entirely on intermo-
lecular interactions.
The molecules specifically responsible for a soap's cleansing properties are typically
salts of fatty acids, which are ionic compounds of the form RCO, Na* or RCO, K*.
In these compounds, R is a long hydrocarbon chain, and the fatty acid salts generally
contain from 12 to 18 carbons. Examples include potassium oleate and sodium
palmitate:
CH3
CH3
Na
H2ha
Sodium palmitate
C16H3102NA
Potassium oleate
C18H3302K
When a soap dissolves in water, it does so as its individual ions: the metal cation
(Na* or K*) and the carboxylate anion (RCO,). Of these two species, the carboxylate
anion is the one that is directly responsible for the soap's cleansing properties, because
it has vastly different characteristics at its two ends (Fig. 2-26). Specifically:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY