Refer to the galvanic cell below (the contents of each half-cell are written beneath each compartment): Pt Cr 0.10 M MnO4 0.40 M Cr*+ 0.20 M Mn²+ 0.30 M Cr2O7²- 0.010 M H* 0.010 M H* The standard reduction potentials are as follows: MnO4¯ + 8H* + 5e Cr2072 + 14H* + 6e → Mn+ + 4H2O, ɛ° = 1.51 V 2Cr3+ + 7H2O, ɛ° = 1.33 V %3D 5. In the balanced cell reaction, what is the stoichiometric coefficient for H*? a. 5 b. 6 c. 30 d. 22 e. 2
Refer to the galvanic cell below (the contents of each half-cell are written beneath each compartment): Pt Cr 0.10 M MnO4 0.40 M Cr*+ 0.20 M Mn²+ 0.30 M Cr2O7²- 0.010 M H* 0.010 M H* The standard reduction potentials are as follows: MnO4¯ + 8H* + 5e Cr2072 + 14H* + 6e → Mn+ + 4H2O, ɛ° = 1.51 V 2Cr3+ + 7H2O, ɛ° = 1.33 V %3D 5. In the balanced cell reaction, what is the stoichiometric coefficient for H*? a. 5 b. 6 c. 30 d. 22 e. 2
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section17.4: Voltaic Cells And Cell Potential
Problem 17.4PSP: Given this reaction, its standard potential, and the standard half-cell potential of 0.34 V for the...
Related questions
Question
100%
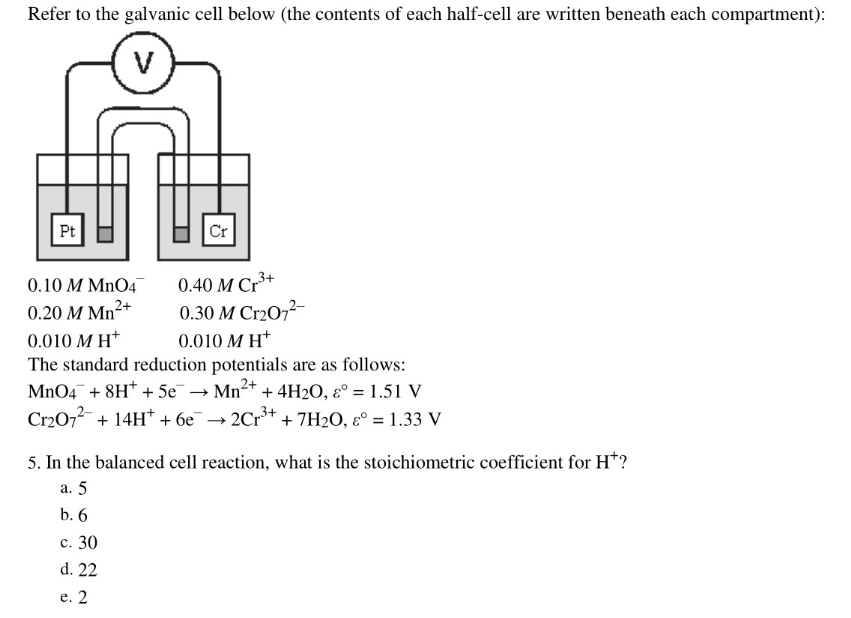
Transcribed Image Text:Refer to the galvanic cell below (the contents of each half-cell are written beneath each compartment):
Pt
Cr
0.10 M MnO4
0.40 M Cr*+
0.20 M Mn²+
0.30 M Cr2O7²-
0.010 M H*
0.010 M H*
The standard reduction potentials are as follows:
MnO4¯ + 8H* + 5e
Cr2072 + 14H* + 6e
→ Mn+ + 4H2O, ɛ° = 1.51 V
2Cr3+
+ 7H2O, ɛ° = 1.33 V
%3D
5. In the balanced cell reaction, what is the stoichiometric coefficient for H*?
a. 5
b. 6
c. 30
d. 22
e. 2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning