Regions of the Electromagnetic Spectrum 20. Use the pentagon shape at the bottom of the figure to slide along the spectrum. In the upper left corner you can see information about the wavelength and frequency of the radiation. Move the pentagon along the spectrum to help you fill in the blank spaces in the table below. You will need to calculate the energy column using the equation you gave in Question 19. The first one (gamma rays) has been done for you as an example. ABLE 10.3 Region Gamma Rays (low end only) X-Rays Ultraviolet Radation Violet Light (high end only) Red Light (low end only) Infrared Radiation Mircowave Radiation Radio Waves (high end only) Wavelength (in m) Low High 1.55 x 10-10 1.3 x 10-8 1 x 10-5 6.53 x 10-¹ 1.42 × 10-8 Frequency (in Hz) Low High 1.94 x 1018 3.55 x 10¹1 1.77 x 1018 1.03 x 1014 4.21 x 108 Energy (in Joules) Low High 1.29 × 10-15
Regions of the Electromagnetic Spectrum 20. Use the pentagon shape at the bottom of the figure to slide along the spectrum. In the upper left corner you can see information about the wavelength and frequency of the radiation. Move the pentagon along the spectrum to help you fill in the blank spaces in the table below. You will need to calculate the energy column using the equation you gave in Question 19. The first one (gamma rays) has been done for you as an example. ABLE 10.3 Region Gamma Rays (low end only) X-Rays Ultraviolet Radation Violet Light (high end only) Red Light (low end only) Infrared Radiation Mircowave Radiation Radio Waves (high end only) Wavelength (in m) Low High 1.55 x 10-10 1.3 x 10-8 1 x 10-5 6.53 x 10-¹ 1.42 × 10-8 Frequency (in Hz) Low High 1.94 x 1018 3.55 x 10¹1 1.77 x 1018 1.03 x 1014 4.21 x 108 Energy (in Joules) Low High 1.29 × 10-15
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 45AP: Suppose an atom in an excited state can return to the ground state in two steps. It first falls to...
Related questions
Question
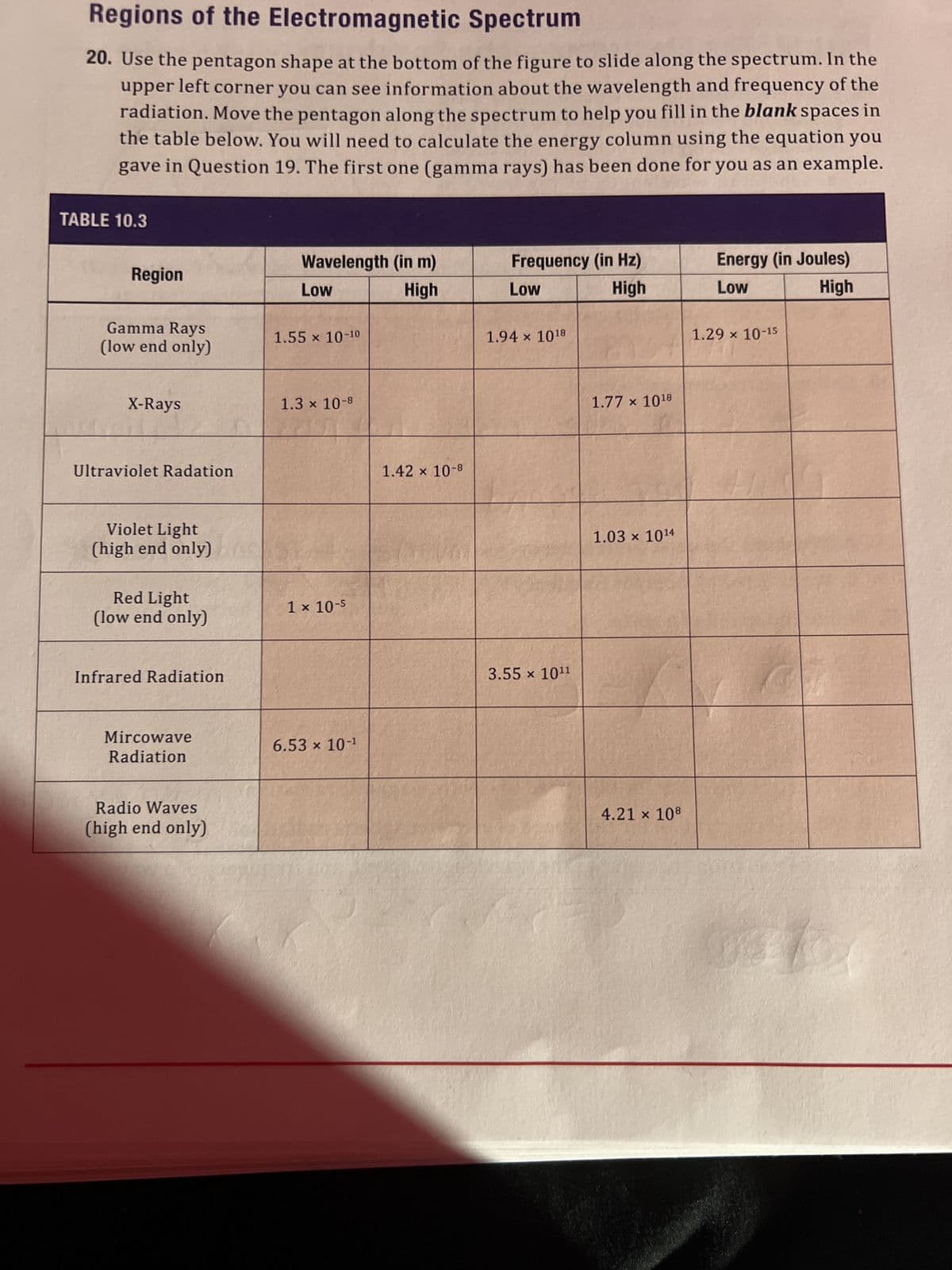
Transcribed Image Text:Regions of the Electromagnetic Spectrum
20. Use the pentagon shape at the bottom of the figure to slide along the spectrum. In the
upper left corner you can see information about the wavelength and frequency of the
radiation. Move the pentagon along the spectrum to help you fill in the blank spaces in
the table below. You will need to calculate the energy column using the equation you
gave in Question 19. The first one (gamma rays) has been done for you as an example.
TABLE 10.3
Region
Gamma Rays
(low end only)
X-Rays
Ultraviolet Radation
Violet Light
(high end only)
Red Light
(low end only)
Infrared Radiation
Mircowave
Radiation
Radio Waves
(high end only)
Wavelength (in m)
Low
High
1.55 × 10-10
1.3 × 10-8
1 x 10-5
6.53 × 10-¹
1.42 × 10-8
Val
Frequency (in Hz)
Low
High
1.94 × 1018
3.55 x 10¹¹
1.77 × 1018
1.03 × 10¹4
4.21 × 108
Energy (in Joules)
Low
High
1.29 × 10-15
BR
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning