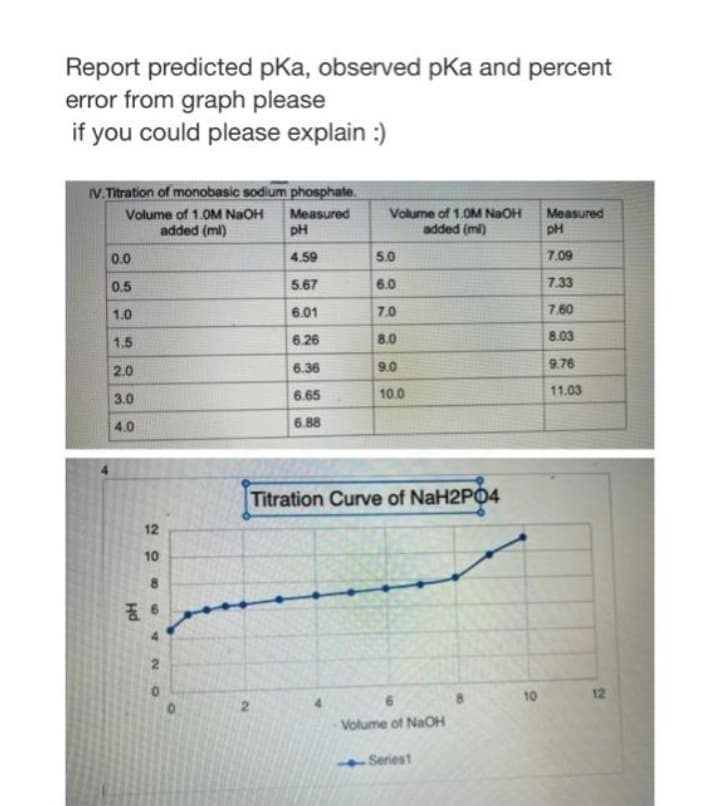
Report predicted pKa, observed pKa and percent error from graph please if you could please explain :) IV. Titration of monobasic sodium phosphate. Volume of 1.0M NaOH added (ml) Measured pH Volume of 1.0M NaOH Measured added (ml) 0.0 4.59 5.0 7.09 0.5 5.67 6.0 7.33 1.0 6.01 7.0 7.60
Q: The Tietjen and Moore paper gives the following set of 8 mass spectrometer measurements on a uranium...
A:
Q: At 400 K, an equilibrium mixture of H2, 12, and HI consists of 0.065 mol H2, 0.079 mol 12, and 0.13 ...
A:
Q: Which of the following is/are true concerning a chemical reaction at equilibrium? 1. The system will...
A: Reversible reaction: The chemical reaction in which the products can react to reform the reactants. ...
Q: Encircle and identify the functional groups
A:
Q: How many moles of NaOH are present in 12.5 mL of 0.210 M NaOH?
A:
Q: Put some water in cup. Sprinkle black pepper all over the surface. What does the pepper do? Tell you...
A: Polar molecules dissolve in polar solvents whereas non polar molecules dissolve in non polar solvent...
Q: . Calculate each of the following: a. the mass of a copper pipe in which the copper has volume of 70...
A:
Q: Question 18 The cell notation for the Galvanic cell is Cd(s)/Ca2*M2(s)/I" The Standard Reduction Pot...
A: This is a redox reaction. In this reaction, Cd oxidised to Cd2+ and I2 reduced to I- . Using Nernst...
Q: Provide the proper IUPAC or common name for the following compound. Br HO Br
A:
Q: Francis A. Santos Which of the following chemical grades are specially manufactured, have exceptiona...
A: Which of the following chemical grades are specially manufactured, have exceptional purity and used ...
Q: What is the cell potential for the following reaction at 25 °C? Ni(s) + Cu2+(0.010 M) → Ni2+(0.001...
A:
Q: The cell notation for the Galvanic cell is Cd(s)/Cd2+//I2(s)/I- The Standard Reductio...
A: Cell notation for the Galvanic cell is :- Cd(s)/Cd2+//I2(s)/I-
Q: Determine the concentration of A in 2A -> product second order reaction after 45 seconds. Use the gi...
A: For a second order reaction, Time, t = 45 seconds Initial concentration of A, Ao = 6.714 M Rate cons...
Q: 15) Which one of the compounds corresponds to this IR spectrum. NH2 1000 2000 3000 4D00
A:
Q: Consider the reaction: BaSO4 Ba2+ + so42-; Ksp = 1.1 x 10-10 What is AG° at 25 °C? 9.3 kJ/mol 1.6 kJ...
A:
Q: 1. SOCI2 2. Et,CuLi 3. (a) LİAIH4 (b) H2O OH
A:
Q: Ammonium phosphate (NH,),PO,) is an important ingredient in many solid fertilizers. It can be made b...
A:
Q: Determine the concentration in mol/L (M) of a solution of 18.25 g of HCl in 250.0 mL of water and id...
A: The molecular weight of HCl is 36.5 gm/mol That 36.5 gm HCl=1mol Hence 18.25 gm HCl=18.25gm×1mol/36....
Q: At 2000 °C, N2(g) + O2(g) 2 NO(g); Kc = 4.10 x 10-4 What is Kp for this reaction? 2.17 x 10-...
A:
Q: 2. Consider a deuterium atom (1 electron, 1 deuteron). Sketch the E vs. B, diagram, including those ...
A: EPR is electron spin resonance which is used to study the species containing unpaired electrons. The...
Q: How much distilled water must be added to a 25.00 mL 0.5104 M aqueous H2SO4 solution to prepare a 0....
A: Given, Volume of aqueous H2SO4 solution = 25.00 mL Concentration of aqueous H2SO4 solution = 0.5104 ...
Q: Describe the four types of information that is provided by a H1 NMR spectra.
A: The information we get from the 1H NMR are discussed as : 1) The number of signal sets The num...
Q: What is the correct way of dealing with acid spills? (A Neutralize with a basic solution before wipi...
A: Concentration acids are highly dangerous and can cause damage to skin when in contact.
Q: Your lab colleague doesn’t understand why you want make a stock solution and then dilute it when you...
A:
Q: Consider the following equilibrium: N204(8) = 2NO2(g) Suppose two different experiments were conduct...
A:
Q: Which of the following glassware is best used for drying solid samples in an oven? A Watch glass B C...
A: Different equipment are used for different purposes in chemical laboratory. Some common equipments ...
Q: After carrying out the following operations, how many significant figures are appropriate to show in...
A: We have to predict the number of Sig figs in result.
Q: Which of the following is the correct IUPAC name for the MAJOR substrate product formed by the dehyd...
A:
Q: (SPM10-29] Which equations represent double decomposition reactions that form a precipitate? Na So. ...
A: Given reactions are : I. CuSO4 + Na2CO3 -------> CuCO3 + Na2SO4 II. CuSO4 + Mg(NO3)2 ------->...
Q: How many grams of gas are present in each of the following cases? b. 8.75 L of C₂H₂ at 378.3 kPa and...
A: We have to predict the mass of gases.
Q: For H,P - HP- + H* • The weak acid H,P dissociates a little and the even weaker acid HP- much less t...
A:
Q: If the populations of two levels of energies E₁ and E, and statistical weights g; and g¡(E¡ < Ej) ar...
A:
Q: One method for the decomposition of carbon dioxide proceeds as follows: 2CO2(g) Which of the followi...
A:
Q: 2. Determine the oxidation numbers of the atoms/ions in each of the following. H2 CIo, ??- NaH Ca F2
A:
Q: Nitrogen (N,) gas and hydrogen (H,) gas react to form ammonia (NH,) gas. Suppose you have 9.0 mol of...
A:
Q: Which of the following is the best option for transferring 20 mL of a solution? A Use a 5mL serologi...
A: BELOW ATTACHED FILE SHOWING THE DETAILS ANSWER . ALL THE BEST
Q: Which of the following is a correct practice in handling reagents? A Return any excess reagent to it...
A: The options given are,
Q: Answer highlighted questions please) A 50.050.0 mL solution of 0.1390.139 M KOHKOH is titrated with...
A: Volume of KOH = 50.0mL or 0.050L Molarity of KOH = 0.139M Number of moles of KOH = 0.050 × 0.139 = ...
Q: Question 4 What is the purpose of the salt bridge in an electrochemical cell? To maintain electroneu...
A: This question is related to electrochemistry. Electrochemistry is branch of chemistry in which oxida...
Q: Consider the reaction: N204(g) 2 NO2(g). What is AS° at 25 °C? AHP, kJ mol-1 AGf, kJ mol-1 NO2 33.85...
A:
Q: Wine goes bad soon after opening because the ethanol (CH,CH,OH) in it reacts with oxygen gas (0,) fr...
A:
Q: In the Flint Water Crisis, trihalomethanes were produced in the new water supply due to the mixing o...
A:
Q: at a certain temperature this reaction follows second-order kinetics with a rate constant of 47.7 M ...
A: Reaction:- 2HI ------> H2 + I2
Q: H* HO OH
A: At first -C=O,take H+,than -OH attack to the carbonyl group. -OH is not good leaving group,so it tak...
Q: The reaction between selenous acid and the iodide ion in acid solution is H2SEO3(aq) + 61 (aq) + 4H*...
A: Answer: To find out the effect of change in concentration of iodide ion on rate of reaction, we need...
Q: A chemist obtained the following data for the percent compound Z in triplicates (n=3) of an insectic...
A: Data obtained by the Chemist:- The percent compound Z in triplicates (n=3) of an insecticide prepara...
Q: Nitrogen dioxide decomposes according to the reaction: 2 NO2(g) 2 NO(g) + O2(g); Kp = 4.48 x 10-13 ...
A: Please find your solution below : Chemical equilibrium is a state in which there is no change in amo...
Q: 2.) Formamide decomposes at high temperature. (a) If 0.186 mole of formamide (HCONH2) dissociates in...
A: The equilibrium reaction given is, a) Given: Moles of HCONH2 taken = 0.186 mol. Volume of flask = 2...
Q: Nitrogen dioxide decomposes according to the reaction: 2 NO2(g) 2 NO(g) + O2(g); Kp = 4.48 x 10-13 ...
A:
Q: Consider the reaction aA + bB dD + eE C = catalyst %3D The rate law is Rate = K[A]°[B]°[C]$ %3D Whic...
A: aA + bB +C -------> dD + eE The rate law of a reaction represents Rate is directly proportional...
Step by step
Solved in 5 steps with 1 images

- You have a sample of approximately 100 mg/L of calcium carbonate and the standardized EDTA has a concentration of 0.00085 M. If you only want to spend approximately 15 mL of the titrant and add 25 mL of sample in the Erlenmeyer flask, can you use it or should you dilute it? the sample?, how much? Solution: No, 29 mL would be needed to titrate the sample, you must dilute it approximately 1: 2. Taking, for example, 25 mL of sample and making up to 50 mL.If the water sample used for titration was buffered with acid instead of alkali, the amount of EDTA required to reach the endpoint will: a. stay constant b. be indeterminatec. increased. decreaseA. Why is the titration of the oxalate in the green salt with KMnO4 performed prior to carrying out the redox titration for iron content? (Why can’t you do them the other way – iron first then oxalate?) B. In a titration using 0.02000 M KMnO4 solution, the following data were obtained: Buret readings: FINAL = 15.31 mL INITIAL = 5.02 mL How many moles of KMnO4 were used?
- A 0.045 0 M solution of HA is 0.60% dissociated. Find pKa for HA.Which of the following statements is true regarding permanganimetry? a) Permanganate solution can oxidize water which is catalyzed by the presence of manganese dioxide or manganese ion. b) The light pink endpoint color for permanganimetry tends to fade with time due to the catalytic reaction. c) Standardization of the permanganate titrant is carried out at elevated temperature with sodium oxalate. d) all of these e) none of these2,5 ml volume has taken from an “hypothetic” solution which includes (3+) Sb and (3+) Fe and at the titration with 0.1004 N KMnO4, the wasted amount has found as 16,4 mL. The other 2,5 mL that has taken, has reduced with Zn after that, this 2,5 mL solution has titrates with the same KMnO4 solution solution and the wasted amount is 26,5mL. With these datas find the %concentrations of the ions at the solution.
- The concentration of a solution of EDTA was determined by standardizing against a solution of Ca2+ prepared from the primary standard CaCO3. A 0.4302g sample of CaCO3 was transferred to a 60mL volumetric flask, dissolved using a minimum of 6 M HCl solution, and diluted to volume. A 44mL portion of this solution was transferred into a 250-mL Erlenmeyer flask and the pH adjusted by adding 5 mL of a pH 10 NH3-NH4 L buffer containing a small amount of Mg2+ EDTA. After adding calmagite as a visual indicator, the solution was titrated with the EDTA, requiring 632mL to reach the end point. Calculate the molar concentration of the titrantIf an Ecell of 0.495V was noted when your cell was set-up. What is thesolution pH?Please show all work. 1. estimate the position of the equivalance point 2. estimate the position of the half-equivalance point 3. calculate the percent error in the experimental pKa value (accepted pKa value is 4.75) 4. Identify the buffering region 5. Identify the volme ranges that corresponed to the initial color and the final color of the indicator Volume pH 0 2.88 0.05 2.9 0.14 2.95 0.55 3.14 1.15 3.35 1.45 3.44 1.97 3.56 2.36 3.63 2.77 3.69 3.17 3.75 3.78 3.84 4.09 3.86 4.59 3.92 5 3.98 5.39 4 5.8 4.03 6.3 4.09 6.6 4.11 6.99 4.13 7.5 4.17 8.01 4.22 8.31 4.23 8.8 4.27 9.2 4.3 9.5 4.3 9.91 4.34 10.53 4.37 10.92 4.4 11.33 4.41 11.63 4.44 12.14 4.47 12.53 4.47 12.94 4.51 13.35 4.52 13.74 4.56 14.14 4.56 14.54 4.59 15.04 4.62 15.33 4.64 15.73 4.65 16.22 4.67 16.62 4.71 17.02 4.72 17.33 4.73 17.73 4.75 18.34 4.79 18.63 4.8 19.02 4.82 19.43 4.84 19.92 4.88 20.33 4.9 20.73 4.92…
- Quantitative analysis of metal ions in pharmaceutical and cosmetic products can be done using direct and indirect complexometric titrations employing concepts of complex formation, masking, and blocking. For example, all Fe3+, Mg2+, Al3+ and Zn2+ can form a complex with EDTA at pH 10. However, at pH 5, Mg-EDTA complex formation is inhibited, whereas at pH 2, only Fe-EDTA complex formation is favored. Knowing these, an analyst tried to determine the % composition of a 0.1000 g sample containing soluble salts of Fe3+, Mg2+, and Al3+. The sample was initially dissolved in 250.0 mL of distilled water. To determine the total ion content of the sample, a 50.00 mL aliquot was buffered to pH 10.00 before adding 50.00 mL of 0.0500 M EDTA, and the excess EDTA was back titrated with 24.18 mL of 0.0750 M standard Zn2+ solution until the EBT endpoint. Another 50.00 mL aliquot was buffered to pH 2.00 and was added with small amount of SCN- producing blood-red Fe(SCN)2+ complex. This was titrated…A. What is the characteristic color of ferric ions in solution? B. Why is the titration of the oxalate in the green salt with KMnO4 performed prior to carrying out the redox titration for iron content? (Why can’t you do them the other way – iron first then oxalate?) C. In a titration using 0.02000 M KMnO4 solution, the following data were obtained: Buret readings: FINAL = 15.31 mL INITIAL = 5.02 mL How many moles of KMnO4 were used?For the complexiometric titration of Ca2 + ions in the shell of the egg sample weighing 62,576 g, the necessary procedures were applied to the egg shells and 100 mL sample was prepared. 1 mL of the prepared sample was taken and diluted to 100 mL, the pH was adjusted, and two drops of EBT indicator were dropped on it. The prepared solution was titrated with 23.6 mL of 0.0095 M EDTA solution. What is the value of the amount of calcium in the sample in terms of% CaCO3? (Ca: 40, C: 12, O: 16 g / mol)

