Required information A 0.560-kg piece of solid lead at 20.0°C is placed into an insulated container holding 0.980 kg of liquid lead at 420°C. The system comes to an equilibrium temperature with no loss of heat to the environment. Ignore the heat capacity of the container. The melting point of lead is 327°C and its heat of fusion is 22.9 kJ/kg and the specific heat of lead is 0.130 kJ/kg K. At equilibrium what will happen to the solid lead? v (Click to select) Some solid lead remains. The solid lead will not melt. All the solid lead becomes liquid.
Required information A 0.560-kg piece of solid lead at 20.0°C is placed into an insulated container holding 0.980 kg of liquid lead at 420°C. The system comes to an equilibrium temperature with no loss of heat to the environment. Ignore the heat capacity of the container. The melting point of lead is 327°C and its heat of fusion is 22.9 kJ/kg and the specific heat of lead is 0.130 kJ/kg K. At equilibrium what will happen to the solid lead? v (Click to select) Some solid lead remains. The solid lead will not melt. All the solid lead becomes liquid.
Chapter5: Temperature And Heat
Section: Chapter Questions
Problem 14P
Related questions
Question
two parts to a question
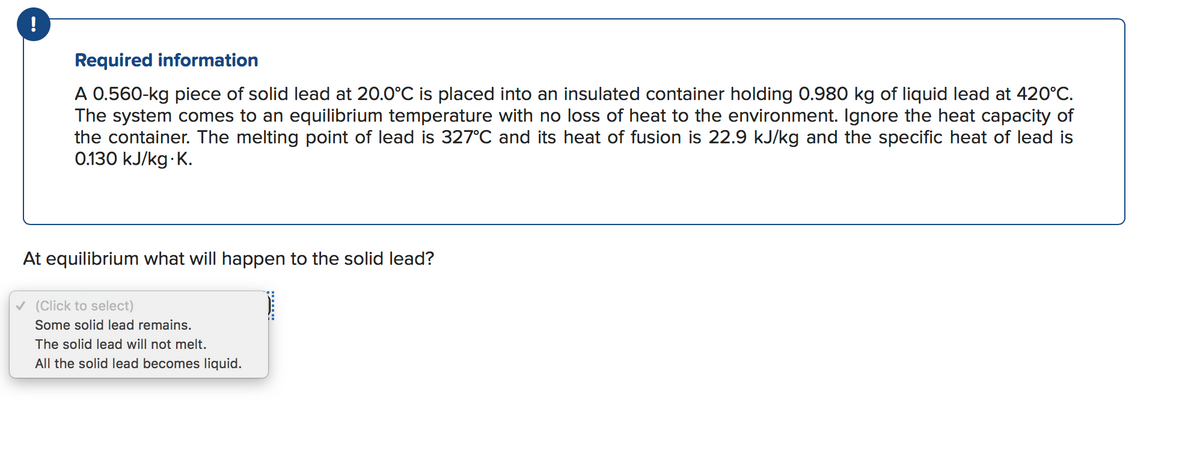
Transcribed Image Text:Required information
A 0.560-kg piece of solid lead at 20.0°C is placed into an insulated container holding 0.980 kg of liquid lead at 420°C.
The system comes to an equilibrium temperature with no loss of heat to the environment. Ignore the heat capacity of
the container. The melting point of lead is 327°C and its heat of fusion is 22.9 kJ/kg and the specific heat of lead is
0.130 kJ/kg K.
At equilibrium what will happen to the solid lead?
v (Click to select)
Some solid lead remains.
The solid lead will not melt.
All the solid lead becomes liquid.

Transcribed Image Text:!
Required information
A 0.560-kg piece of solid lead at 20.0°C is placed into an insulated container holding 0.980 kg of liquid lead at 420°C.
The system comes to an equilibrium temperature with no loss of heat to the environment. Ignore the heat capacity of
the container. The melting point of lead is 327°C and its heat of fusion is 22.9 kJ/kg and the specific heat of lead is
0.130 kJ/kg K.
What is the final temperature of the system?
1°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College