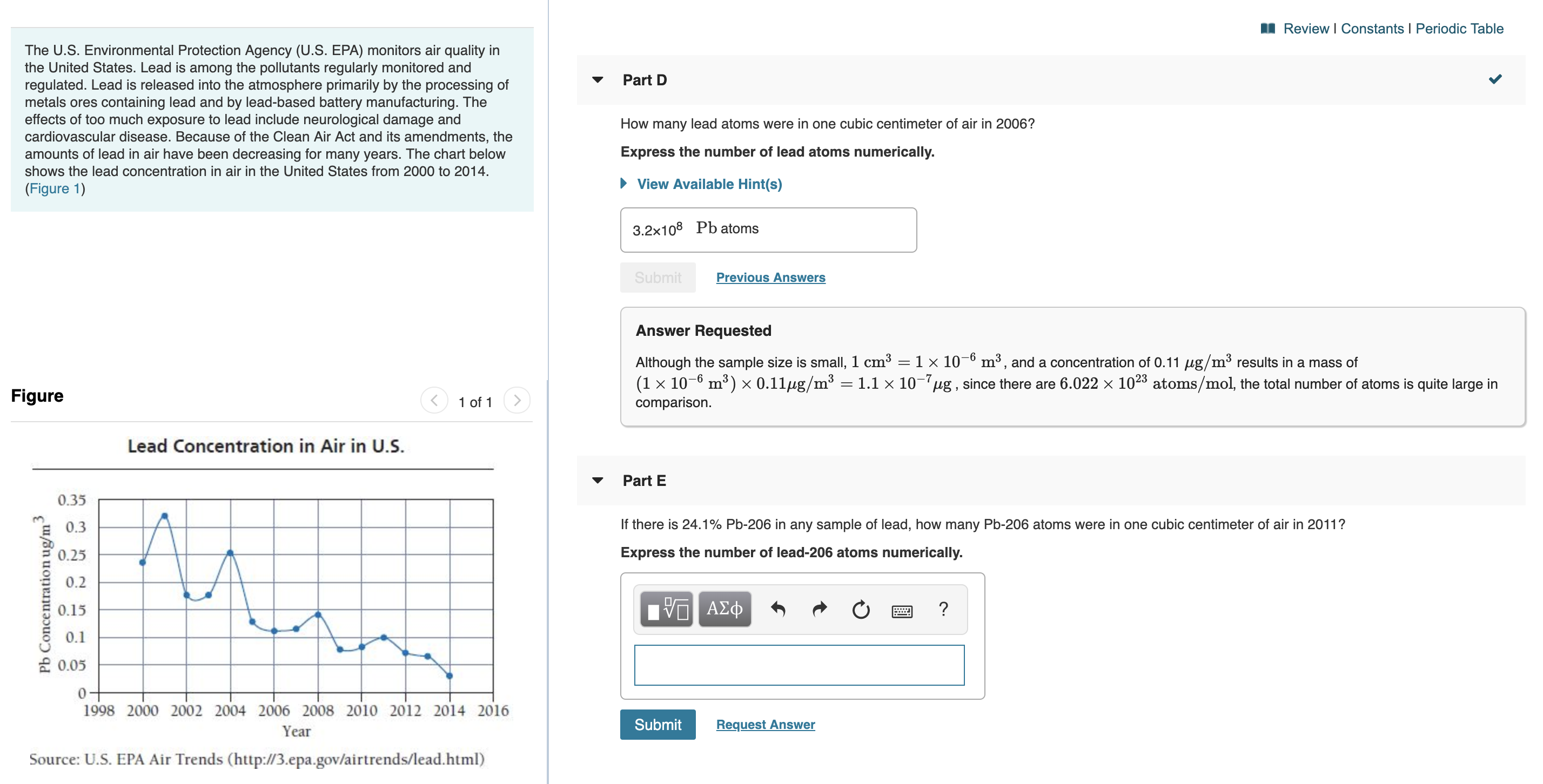
Review I Constants I Periodic Table The U.S. Environmental Protection Agency (U.S. EPA) monitors air quality in the United States. Lead is among the pollutants regularly monitored and regulated. Lead is released into the atmosphere primarily by the processing of metals ores containing lead and by lead-based battery manufacturing. The effects of too much exposure to lead include neurological damage and cardiovascular disease. Because of the Clean Air Act and its amendments, the amounts of lead in air have been decreasing for many years. The chart below shows the lead concentration in air in the United States from 2000 to 2014. Part D How many lead atoms were in one cubic centimeter of air in 2006? Express the number of lead atoms numerically. View Available Hint(s) (Figure 1) 3.2x108 Pb atoms Previous Answers Submit Answer Requested Although the sample size is small, 1 cm3 = 1 x 10-° m3, and a concentration of 0.11 ug/m3 results in a mass of (1 x 10- m3) x 0.11ug/m3 1.1 x 10ug, since there are 6.022 x 1023 atoms/mol, the total number of atoms is quite large in Figure 1 of 1 comparison. Lead Concentration in Air in U.S. Part E 0.35 If there is 24.1% Pb-206 in any sample of lead, how many Pb-206 atoms were in one cubic centimeter of air in 2011? 0.3 Express the number of lead-206 atoms numerically. 0.25 0.2 | ν ΑΣφ ? 0.15 0.1 0.05 0- 1998 2000 2002 2004 2006 2008 2010 2012 2014 2016 Submit Request Answer Year Source: U.S. EPA Air Trends (http://3.epa.gov/airtrends/lead.html) Pb Concentration ug/m
Electronic Effects
The effect of electrons that are located in the chemical bonds within the atoms of the molecule is termed an electronic effect. The electronic effect is also explained as the effect through which the reactivity of the compound in one portion is controlled by the electron repulsion or attraction producing in another portion of the molecule.
Drawing Resonance Forms
In organic chemistry, resonance may be a mental exercise that illustrates the delocalization of electrons inside molecules within the valence bond theory of octet bonding. It entails creating several Lewis structures that, when combined, reflect the molecule's entire electronic structure. One Lewis diagram cannot explain the bonding (lone pair, double bond, octet) elaborately. A hybrid describes a combination of possible resonance structures that represents the entire delocalization of electrons within the molecule.
Using Molecular Structure To Predict Equilibrium
Equilibrium does not always imply an equal presence of reactants and products. This signifies that the reaction reaches a point when reactant and product quantities remain constant as the rate of forward and backward reaction is the same. Molecular structures of various compounds can help in predicting equilibrium.
Please answer Part E
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images









