Rewrite the following balanced equations as net ionic equations. (a) Na,SO4(aq) + BaCl2(aq) → BaSO4(s) + 2 NaCl(aq) (b) 6 NaOH(aq) + 3 Cl2(g) - NaCIO3(aq) + 5 NaCl(aq) + 3 H,0(€) (c) Hg2(NO3)2(aq) + 2 KI(aq) → Hg,I,(s) + 2 KNO3(aq) (d) 3 NaOCI(aq) + KI(aq) → NAIO3(aq) + 2 NaCl(aq) + KCl(aq)
Rewrite the following balanced equations as net ionic equations. (a) Na,SO4(aq) + BaCl2(aq) → BaSO4(s) + 2 NaCl(aq) (b) 6 NaOH(aq) + 3 Cl2(g) - NaCIO3(aq) + 5 NaCl(aq) + 3 H,0(€) (c) Hg2(NO3)2(aq) + 2 KI(aq) → Hg,I,(s) + 2 KNO3(aq) (d) 3 NaOCI(aq) + KI(aq) → NAIO3(aq) + 2 NaCl(aq) + KCl(aq)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 94QAP: A 100.0-g mixture made up of NaCl03, Na2CO3, NaCl, and NaHCO3 is heated, producing 5.95 g of oxygen,...
Related questions
Question
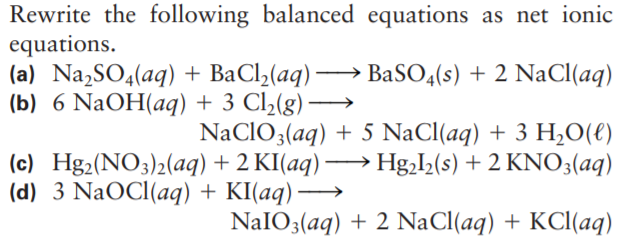
Transcribed Image Text:Rewrite the following balanced equations as net ionic
equations.
(a) Na,SO4(aq) + BaCl2(aq) → BaSO4(s) + 2 NaCl(aq)
(b) 6 NaOH(aq) + 3 Cl2(g)
-
NaCIO3(aq) + 5 NaCl(aq) + 3 H,0(€)
(c) Hg2(NO3)2(aq) + 2 KI(aq) → Hg,I,(s) + 2 KNO3(aq)
(d) 3 NaOCI(aq) + KI(aq) →
NAIO3(aq) + 2 NaCl(aq) + KCl(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning