Road Map Mass (kg) of SiO2 1. convert kg to g 2. divide by M (g/mol) Amount (mol) of SiO2 molar ratio Amount (mol) of SIC 1. multiply by (g/mol) 2. convert g to kg Mass (kg) of SiC Eq. 3.8 % Yield of Sic
Problem Silicon carbide (SiC) is an important ceramic material made by reacting sand (silicon dioxide, SiO2) with powdered carbon at a high temperature. Carbon monoxide is also formed. When 100.0 kg of sand is processed, 51.4 kg of SiC is recovered. What is the percent yield of SiC from this process?
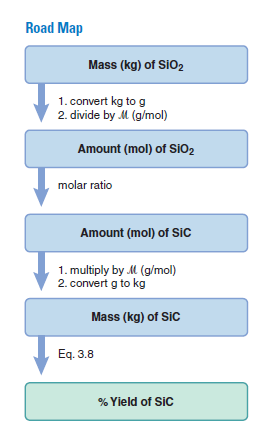
Plan We are given the actual yield of SiC (51.4 kg), so we need the theoretical yield to calculate the percent yield. After writing the balanced equation, we convert the given mass of SiO2 (100.0 kg) to amount (mol). We use the molar ratio to find the amount of SiC formed and convert it to mass (kg) to obtain the theoretical yield. Then, we use as shown to find the percent yield (see the road map).
Trending now
This is a popular solution!
Step by step
Solved in 3 steps








