Part A Calculate the theoretical yield of C,H;Cl when 140 g of C,Hs reacts with 228 g of Cl2, assuming that C, H6 and Cl2 react only to form C2H;Cl and HCI. ? m= g Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Review your calculations and make sure you round to 3 significant figures in the last step. Part B Calculate the percent yield of C,H;Cl if the reaction produces 179 g of C,H;CI. ? percent yield = % Submit Request Answer
Part A Calculate the theoretical yield of C,H;Cl when 140 g of C,Hs reacts with 228 g of Cl2, assuming that C, H6 and Cl2 react only to form C2H;Cl and HCI. ? m= g Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Review your calculations and make sure you round to 3 significant figures in the last step. Part B Calculate the percent yield of C,H;Cl if the reaction produces 179 g of C,H;CI. ? percent yield = % Submit Request Answer
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 40QAP
Related questions
Question
100%
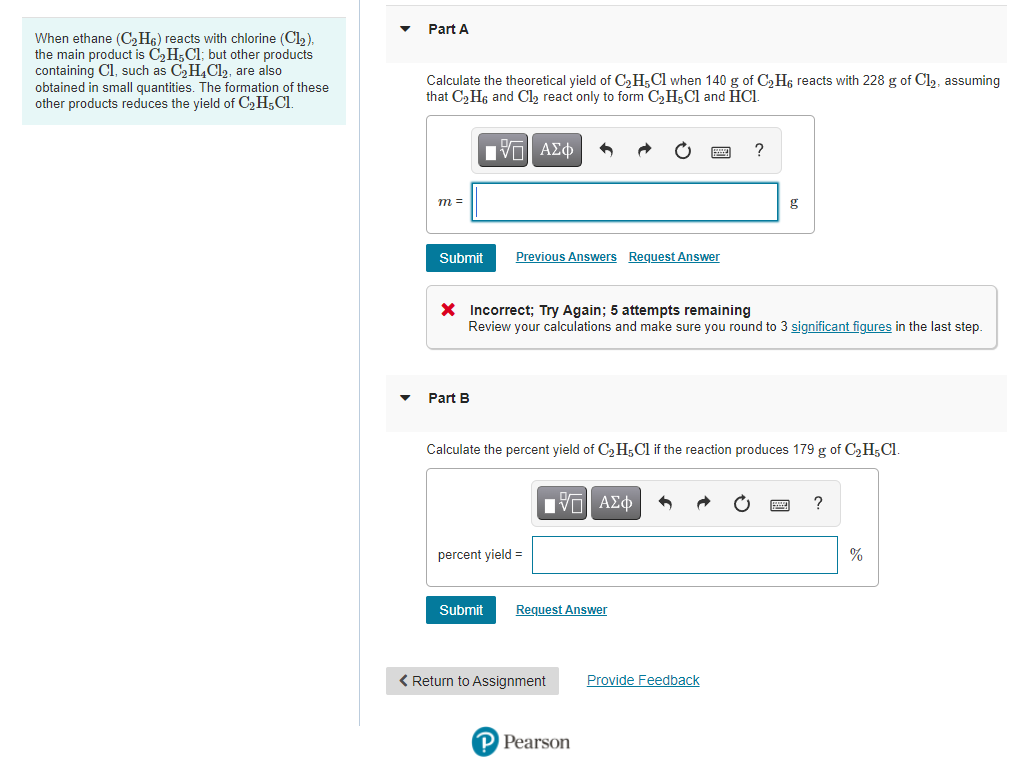
Transcribed Image Text:Part A
When ethane (C2H6) reacts with chlorine (Cl2).
the main product is C,H;Cl; but other products
containing Cl, such as C,H4C12, are also
obtained in small quantities. The formation of these
other products reduces the yield of C,H;Cl.
Calculate the theoretical yield of C2H;Cl when 140 g of C2H6 reacts with 228 g of Cl2, assuming
that C2 H6 and Cl, react only to form C2H;Cl and HCI.
ΑΣφ
?
m =
g
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
Review your calculations and make sure you round to 3 significant figures in the last step.
Part B
Calculate the percent yield of C2 H;Cl if the reaction produces 179 g of C2H;Cl.
Πνα ΑΣφ
?
percent yield =
%
Submit
Request Answer
< Return to Assignment
Provide Feedback
P Pearson
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning