Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter8: Reaction Rates And Equilibrium
Section: Chapter Questions
Problem 8.81E
Related questions
Question
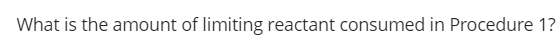
Transcribed Image Text:What is the amount of limiting reactant consumed in Procedure 1?
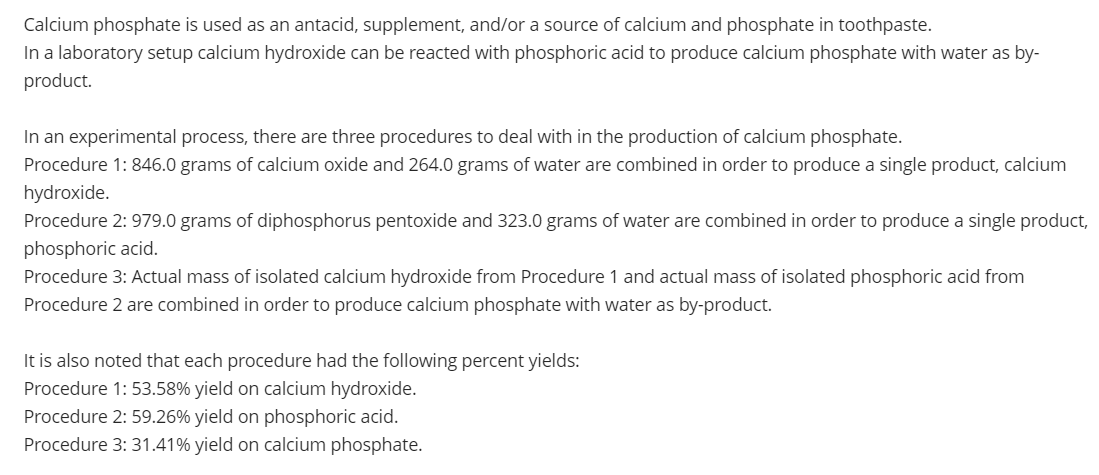
Transcribed Image Text:Calcium phosphate is used as an antacid, supplement, and/or a source of calcium and phosphate in toothpaste.
In a laboratory setup calcium hydroxide can be reacted with phosphoric acid to produce calcium phosphate with water as by-
product.
In an experimental process, there are three procedures to deal with in the production of calcium phosphate.
Procedure 1: 846.0 grams of calcium oxide and 264.0 grams of water are combined in order to produce a single product, calcium
hydroxide.
Procedure 2: 979.0 grams of diphosphorus pentoxide and 323.0 grams of water are combined in order to produce a single product,
phosphoric acid.
Procedure 3: Actual mass of isolated calcium hydroxide from Procedure 1 and actual mass of isolated phosphoric acid from
Procedure 2 are combined in order to produce calcium phosphate with water as by-product.
It is also noted that each procedure had the following percent yields:
Procedure 1: 53.58% yield on calcium hydroxide.
Procedure 2: 59.26% yield on phosphoric acid.
Procedure 3: 31.41% yield on calcium phosphate.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry In Focus
Chemistry
ISBN:
9781305084476
Author:
Tro, Nivaldo J., Neu, Don.
Publisher:
Cengage Learning