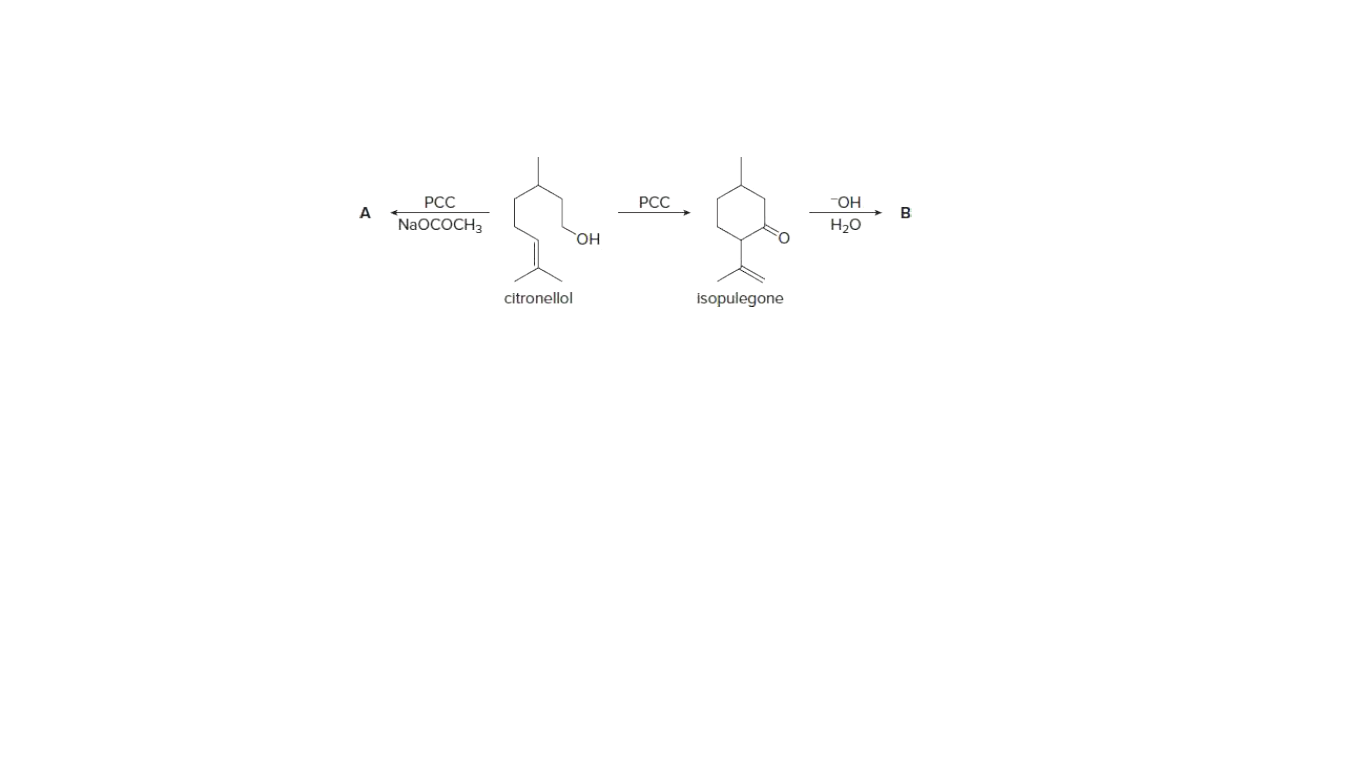
Oxidation of citronellol, a constituent of rose and geranium oils, with PCC in the presence of added NaOCOCH3 forms compound A. A has a
molecular ion in its mass spectrum at 154 and a strong peak in its IR
spectrum at 1730 cm−1, in addition to C—H stretching absorptions.
Without added NaOCOCH3, oxidation of citronellol with PCC yields
isopulegone, which is then converted to B with aqueous base. B has a
molecular ion at 152 and a peak in its IR spectrum at 1680 cm−1, in
addition to C—H stretching absorptions.
a.) Identify the structures of A and B.
b.) Draw a mechanism for the conversion of citronellol to isopulegone.
c.) Draw a mechanism for the conversion of isopulegone to B.
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 6 images









