(S) Indicate the calculation of how many grams of aluminum oxide (Al2O3) would be produced from the reaction of 0.25 grams of aluminum reacting with excess oxygen gas. 4AI (s) + 302 (g)→ 2A1203 (s)
(S) Indicate the calculation of how many grams of aluminum oxide (Al2O3) would be produced from the reaction of 0.25 grams of aluminum reacting with excess oxygen gas. 4AI (s) + 302 (g)→ 2A1203 (s)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter21: The Chemistry Of The Main Group Elements
Section: Chapter Questions
Problem 119IL
Related questions
Question
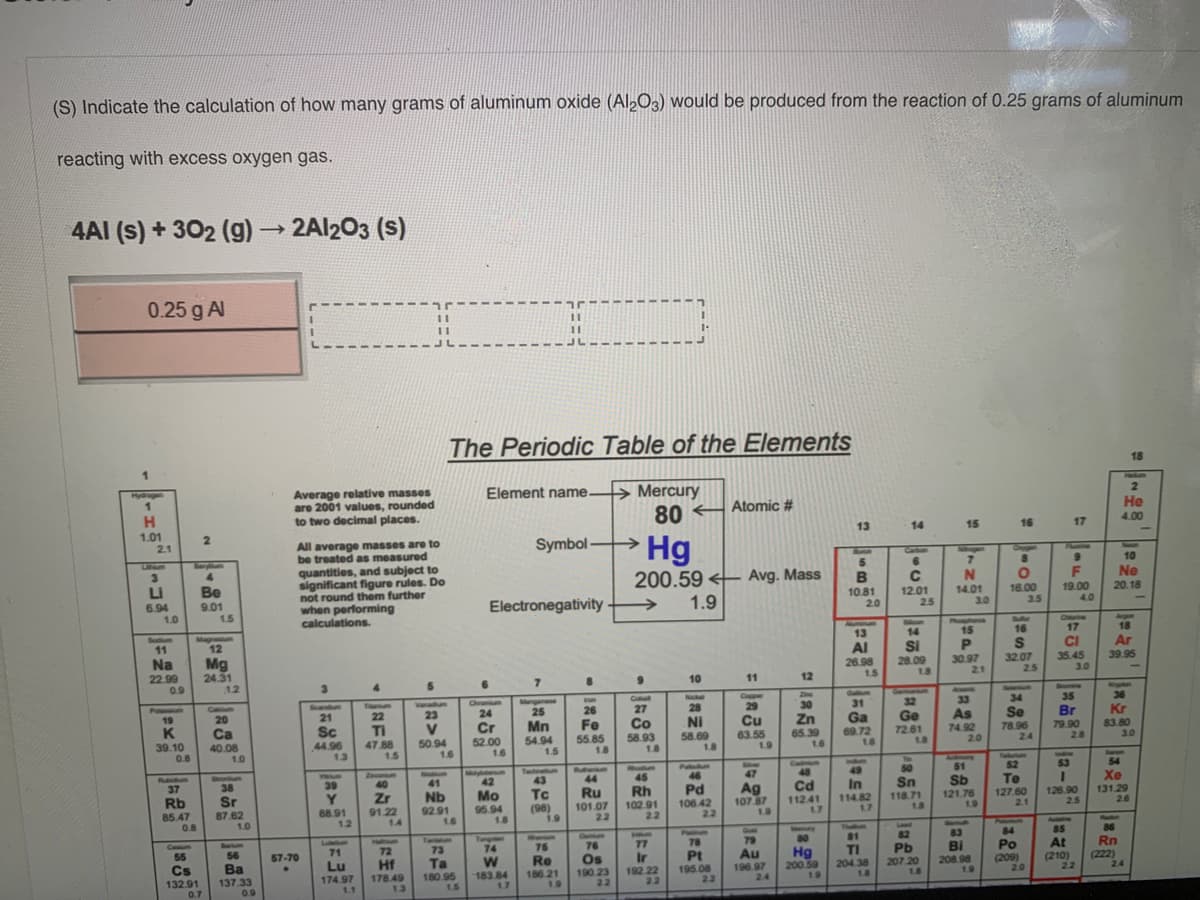
Transcribed Image Text:(S) Indicate the calculation of how many grams of aluminum oxide (Al2O3) would be produced from the reaction of 0.25 grams of aluminum
reacting with excess oxygen gas.
4AI (s) + 302 (g) 2A1203 (s)
0.25 g Al
3D
The Periodic Table of the Elements
18
→ Mercury
80 Atomic #
Hyroge
Pedum
Average relative masses
are 2001 values, rounded
to two decimal places.
Element name
2
He
4.00
H
1.01
2.1
13
14
15
16
17
All average masses are to
be treated as measured
quantities, and subject to
significant figure rules. Do
not round them further
when performing
calculations.
Symbol -
Hg
LINe
Tao
Carbon
Oepn
Renne
Lium
10
4.
Be
200.59 < Avg. Mass
1.9
F
Ne
B
10.81
20
C
12.01
2.5
Li
%3D
16.00
3.5
19.00
4.0
20.18
6.94
1.0
9.01
1.5
Electronegativity
->
14.01
3.0
Ston
14
Alumen
Cherine
Avgan
Sodum
11
Magam
12
Phosghon
15
13
16
17
18
Si
CI
Ar
Na
22.99
0.9
Mg
24.31
12
Al
26.98
P.
30.97
21
32.07
2.5
35.45
3.0
39.95
28.09
18
10
11
12
1.5
4.
Geenartams
Awc
Copper
29
Gatfen
Sxandu
21
Tanium
22
Calcium
Vanadum
Chram
Cua
30
32
33
34
35
36
31
Ga
69.72
1.6
26
27
28
19
K
39.10
0.8
20
23
24
25
Kr
Se
78.96
24
Br
Fe
55.85
1.8
Co
58.93
1.8
NI
58.69
1.8
Cu
63.55
1.9
Zn
65.39
1.6
Ge
72.61
1.3
As
74.92
2.0
Sc
Ti
47.88
1.5
Cr
52.00
1.6
Ca
V
Mn
40.08
1.0
44.96
13
50.94
1.6
54.94
1.5
79.90
2.8
83.80
3.0
Autonuns
51
Sb
121.76
1.9
Takuu
52
Sanan
Ca
48
In
Mayn
42
Sentun
Mmeadhums
Testnaum
43
Ruterkum
44
Pulun
Rubidum
37
Ddium
38
Sr
Notoon
41
47
53
54
50
Sn
39
40
45
46
49
Cd
112.41
Te
127.60
2.1
Xe
131.29
26
In
Zr
91.22
Nb
92.91
1.6
Ru
101.07
22
Rh
102.91
22
Pd
106.42
22
Ag
107.87
1.9
Mo
Tc
Rb
85.47
0.8
87.62
1.0
Y
88.91
12
95.94
1.8
(98)
1.9
114.82
17
118.71
1.8
126.90
2.5
17
14
Damay
Autadine
Rasdon
Thuiuns
81
TI
Loed
Mesoan
Cumian
Palma
Ta m
73
Tungten
74
Cestum
Lubtm
76
78
79
80
82
83
84
85
86
72
75
77
55
Cs
132.91
0.7
56
57-70
71
At
Rn
Pb
207 20
Po
(209)
2.0
Bi
Os
190.23
22
Ir
192 22
22
Pt
195.08
22
Au
196.97
Hg
200.59
19
Hf
Ta
Re
186 21
1.9
Ba
137.33
0.9
Lu
174.97
178.49
13
180.95
1.5
183.84
1.7
204.38
1.8
208.98
19
(210)
22
(222)
24
2.4
1.8
1.1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax