s.com/course.html?courseld%3D15828855&HeplD353706194671Of71a192e43597b01c6e0#10301 Principles of Chemistry I (Chem 1107-327) signments <09 - Pre-Lab 9.11 Pre-Lab Methane burns in oxygen to produce CO2 and H2O. CH:(9) + 202(g)→2H»O(1) +CO2(g) Part A If 0.55 L of gaseous CH, is bumed at STP, what volume of O, is required for complete combustion? Hνα ΑΣφ Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining 40 DELL
s.com/course.html?courseld%3D15828855&HeplD353706194671Of71a192e43597b01c6e0#10301 Principles of Chemistry I (Chem 1107-327) signments <09 - Pre-Lab 9.11 Pre-Lab Methane burns in oxygen to produce CO2 and H2O. CH:(9) + 202(g)→2H»O(1) +CO2(g) Part A If 0.55 L of gaseous CH, is bumed at STP, what volume of O, is required for complete combustion? Hνα ΑΣφ Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining 40 DELL
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
Transcribed Image Text:s.com/course.html?courseld%3D15828855&HeplD353706194671Of71a192e43597b01c6e0#10301
Principles of Chemistry I (Chem 1107-327)
signments
<09 - Pre-Lab
9.11 Pre-Lab
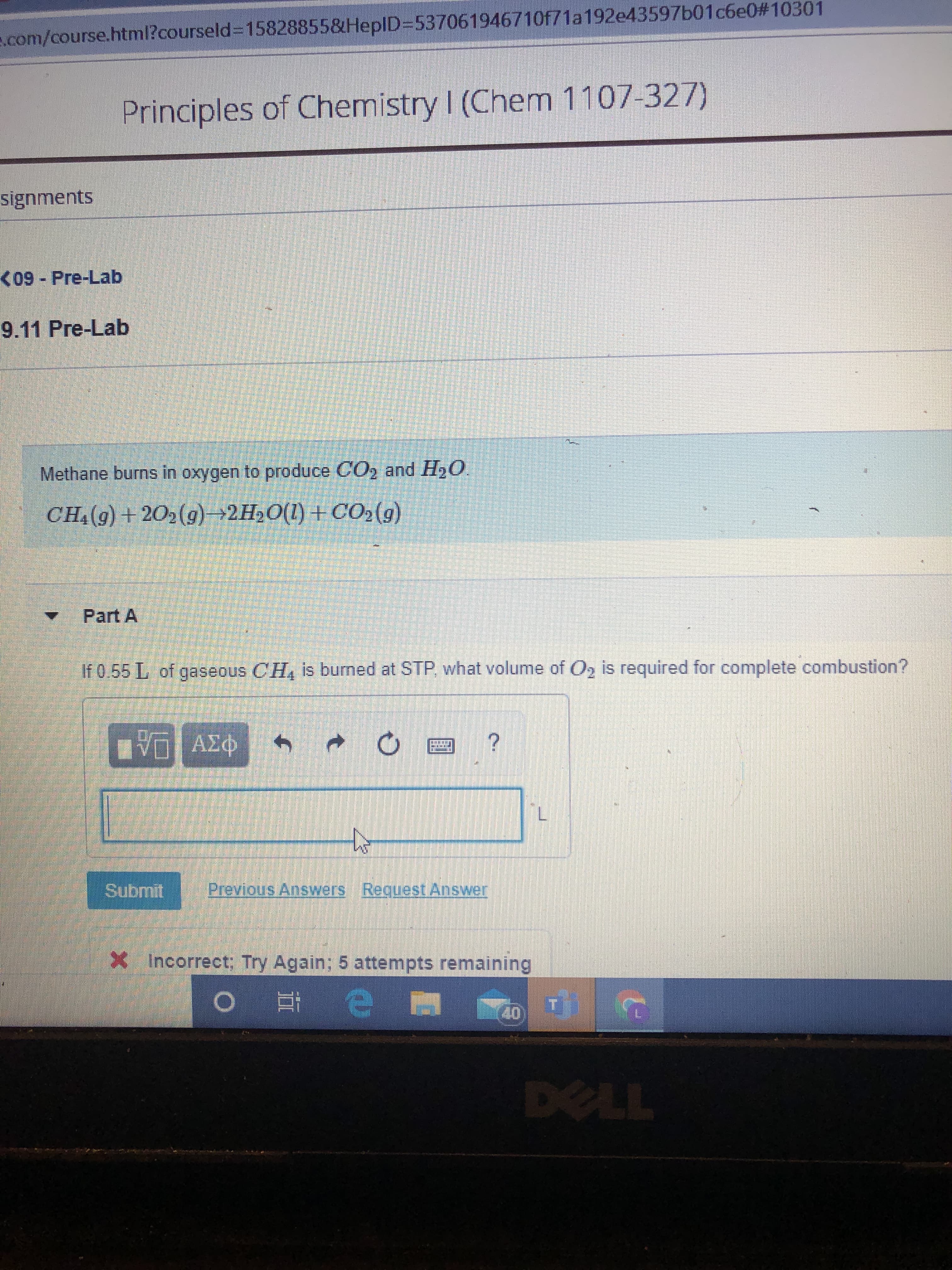
Methane burns in oxygen to produce CO2 and H2O.
CH:(9) + 202(g)→2H»O(1) +CO2(g)
Part A
If 0.55 L of gaseous CH, is bumed at STP, what volume of O, is required for complete combustion?
Hνα ΑΣφ
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
40
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

