4. Write the formulas for the following ionic compounds. Please show the cation and polyatomic ion charges as part of your work You may use the table below for reference. Name acetate bicarbonate carbonate hydroxide a Manganese (IV) nitrite b. Copper (II) phosphate c. Iron (III) carbonate d Aluminum hydroxide C Zinc sulfate f. Cobalt (III) acetate Formula C₂H₂O₂ HCO CO₂ OH Name nitrite phosphate sulfate sulfite Formula NOT PO SO SO,²
4. Write the formulas for the following ionic compounds. Please show the cation and polyatomic ion charges as part of your work You may use the table below for reference. Name acetate bicarbonate carbonate hydroxide a Manganese (IV) nitrite b. Copper (II) phosphate c. Iron (III) carbonate d Aluminum hydroxide C Zinc sulfate f. Cobalt (III) acetate Formula C₂H₂O₂ HCO CO₂ OH Name nitrite phosphate sulfate sulfite Formula NOT PO SO SO,²
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter10: The Mole
Section: Chapter Questions
Problem 179A
Related questions
Question
Please complete, asap!!!
Need all subparts till e"
As it's a not a lengthy qs.
Will provide helpful ratings for correct solution. Thank u
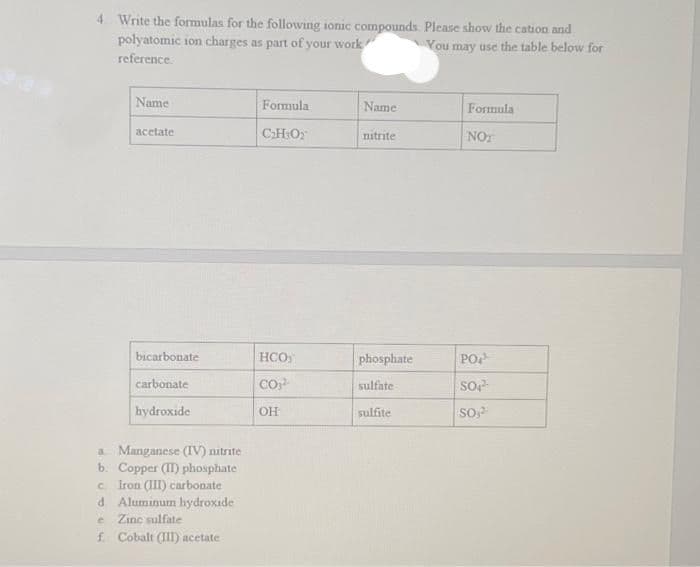
Transcribed Image Text:4. Write the formulas for the following ionic compounds. Please show the cation and
polyatomic ion charges as part of your work
You may use the table below for
reference.
Name
acetate
bicarbonate
carbonate
hydroxide
a Manganese (IV) nitrite
b. Copper (II) phosphate
C Iron (III) carbonate
d. Aluminum hydroxide
Zinc sulfate
e
f. Cobalt (III) acetate
Formula
C₂H₂O₂
HCO
CO²-
OH
Name
nitrite
phosphate
sulfate
sulfite
Formula
NOT
PO
SO₂²
SO,2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning