Sample pH Color with FeCla 0.15% salicylic acid 3.0 Dark Purple Commercial aspirin 4.0 Dark purple-pink Buffered aspirin Aspirin product 6.0 Light purple-pink 5.0 Pink 3. Compare the purity of the three aspirin samples with that of the reference sample (0.15 % salicylic acid). Which sample is the least pure? Which sample is the purest? Explain how you made your choices.
Sample pH Color with FeCla 0.15% salicylic acid 3.0 Dark Purple Commercial aspirin 4.0 Dark purple-pink Buffered aspirin Aspirin product 6.0 Light purple-pink 5.0 Pink 3. Compare the purity of the three aspirin samples with that of the reference sample (0.15 % salicylic acid). Which sample is the least pure? Which sample is the purest? Explain how you made your choices.
Chapter5: Errors In Chemical Analyses
Section: Chapter Questions
Problem 5.10QAP
Related questions
Question
Compare the purity of the three aspirin samples with that of the reference sample (0.15 % salicylic acid). Which sample is the least pure? Which sample is the purest? Explain how you made your choices
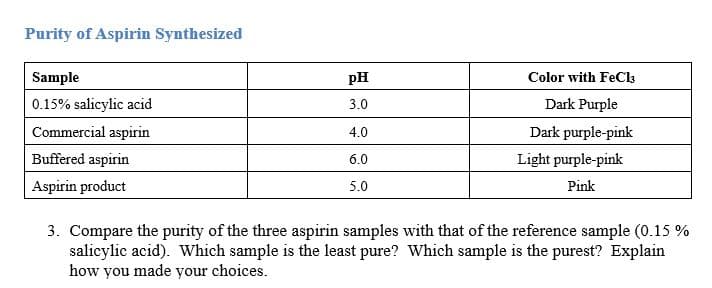
Transcribed Image Text:Sample
pH
Color with FeCla
0.15% salicylic acid
3.0
Dark Purple
Commercial aspirin
4.0
Dark purple-pink
Buffered aspirin
Aspirin product
6.0
Light purple-pink
5.0
Pink
3. Compare the purity of the three aspirin samples with that of the reference sample (0.15 %
salicylic acid). Which sample is the least pure? Which sample is the purest? Explain
how you made your choices.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



