Mass of NaCl needed for 50.0 mL of 0.200 M NaCl solution. (Show calculation below.) Change in pH from initial pH after addition of Change in pH from initial Initial pH pH after addition of strong acid strong base 7.05 .45 7.092.47 4.62456 phosphate lo.75 .70 Water 11.89 0.200 M NaCl acetate 4.70 buffer buffer L6,70 QUESTIONS 1. Do chloride or sodium ions have a big effect on pH? What is the evidence that supports your answer? (Hint: Compare the initial pH's of water and 0.200 M NaCl.) 2. Is the 0.200 M NaCl solution buffered or not? What is the evidence that supports your answer?
Mass of NaCl needed for 50.0 mL of 0.200 M NaCl solution. (Show calculation below.) Change in pH from initial pH after addition of Change in pH from initial Initial pH pH after addition of strong acid strong base 7.05 .45 7.092.47 4.62456 phosphate lo.75 .70 Water 11.89 0.200 M NaCl acetate 4.70 buffer buffer L6,70 QUESTIONS 1. Do chloride or sodium ions have a big effect on pH? What is the evidence that supports your answer? (Hint: Compare the initial pH's of water and 0.200 M NaCl.) 2. Is the 0.200 M NaCl solution buffered or not? What is the evidence that supports your answer?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.58QE
Related questions
Question
THIS IS 1 OF 2
I'm uploading the other 2 images that go with these next, because the limit shows 2 images
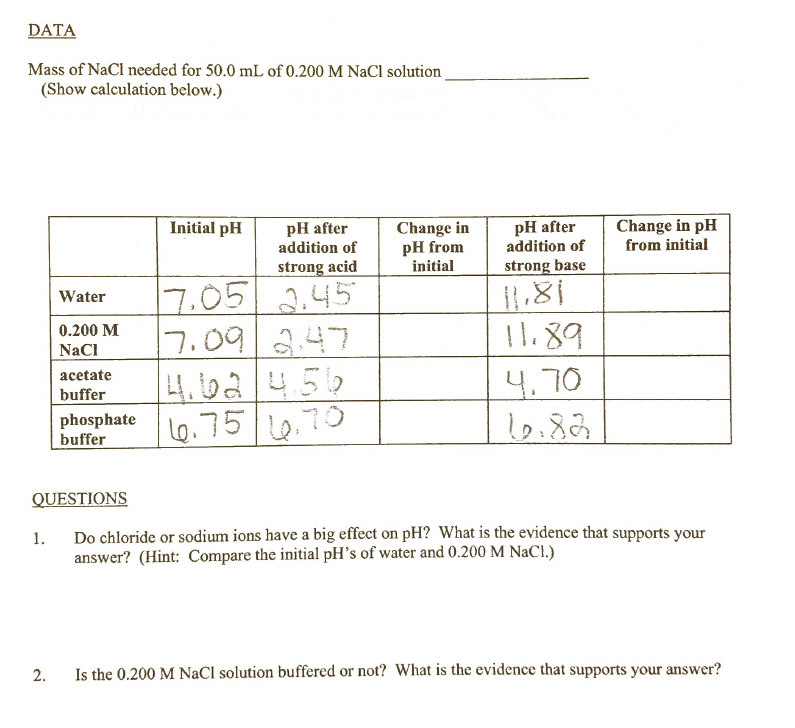
Transcribed Image Text:DATA
Mass of NaCl needed for 50.0 mL of 0.200 M NaCl solution
(Show calculation below.)
pH after
addition of
Change in pH
from initial
Initial pH
pH after
addition of
Change in
pH from
initial
strong acid
strong base
7.05
2.45
Water
I1.89
17.092.47
4.62/456
Lo.75 0.70
0.200 M
NaCl
acetate
4.70
buffer
phosphate
buffer
QUESTIONS
Do chloride or sodium ions have a big effect on pH? What is the evidence that supports your
answer? (Hint: Compare the initial pH's of water and 0.200 M NaCl.)
1.
2.
Is the 0.200 M NaCl solution buffered or not? What is the evidence that supports your answer?

Transcribed Image Text:Saline is another name for a solution containing sodium chloride. Severely dehydrated patients
may be given a buffercd saline solution intravenously to restore their fluid balance. Do you think
that a buffered saline solution would contain only sodium chloride in the solution? Justify your
answer using your experimental evidence.
3.
4.
Use the dilution equation to calculate the number of milliliters of 3.00 M NaCl needed to make
600.0 mL of a saline solution with a final sodium chloride concentration of 0.154 M. Show your
work.
5.
Calculate the molarity of a solution made by dissolving 85.0 grams of Na;SO4 in enough water to
make 250.0 mL of solution. Show your work.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning