Schottky defect Frenkel defect When Frenkel defect occurs, there is n change in charge because the cation maintains the same positive charge as an interstitial. Frenkel defect involves a cation- vacancy and a cation-interstitial pair. Schottky defect involves a cation vacancy and an anion vacancy pair. When Schottky defect occurs, the charge neutrality of the crystal is not maintained because it involves both anions and cations.
Schottky defect Frenkel defect When Frenkel defect occurs, there is n change in charge because the cation maintains the same positive charge as an interstitial. Frenkel defect involves a cation- vacancy and a cation-interstitial pair. Schottky defect involves a cation vacancy and an anion vacancy pair. When Schottky defect occurs, the charge neutrality of the crystal is not maintained because it involves both anions and cations.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter21: The Solid State: Crystals
Section: Chapter Questions
Problem 21.28E
Related questions
Question
33
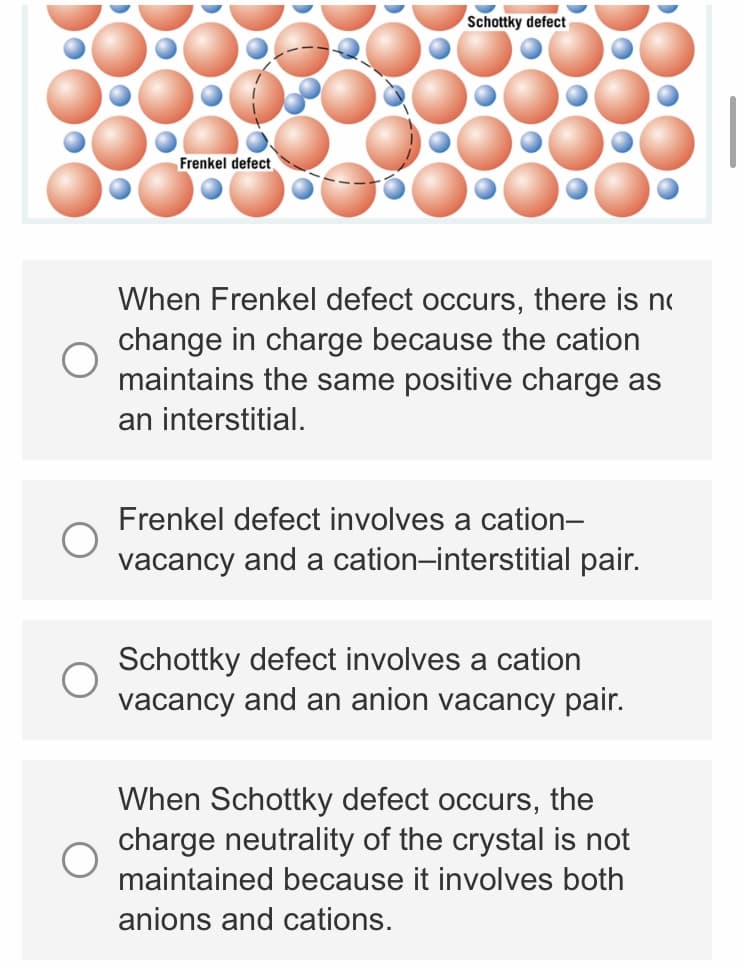
Transcribed Image Text:Schottky defect
Frenkel defect
When Frenkel defect occurs, there is no
change in charge because the cation
maintains the same positive charge as
an interstitial.
Frenkel defect involves a cation-
vacancy and a cation-interstitial pair.
Schottky defect involves a cation
vacancy and an anion vacancy pair.
When Schottky defect occurs, the
charge neutrality of the crystal is not
maintained because it involves both
anions and cations.

Transcribed Image Text:3
Which one below is not true for Frenkel
and Schottky defects in ionic solids? *
Schottky defect
Frenkel defect
When Frenkel defect occurs, there is no
change in charge because the cation
maintains the same positive charge as
an interstitial.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning