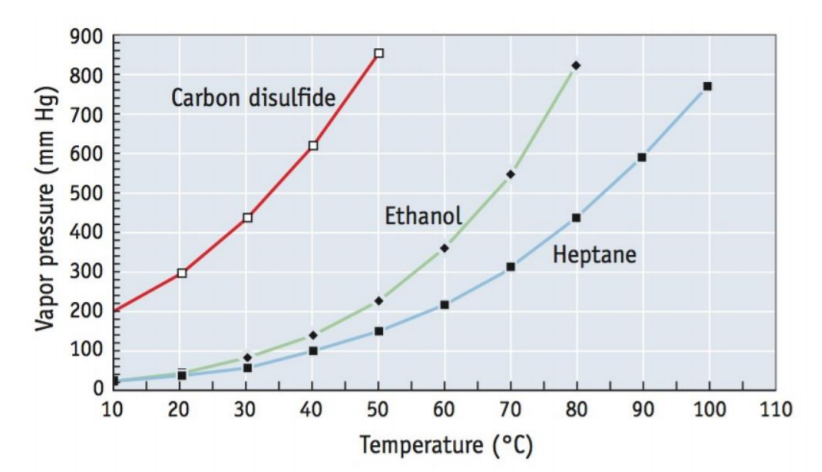
se the vapour pressure curves in the graph, how can I determine the following: a) What is the vapour pressure of ethanol at 60 degrees C? b) At what temperature does heptane ( C7H16) have a vapour pressure of 500 mm Hg? c) What are the approximate boiling points of each of the 3 substances? d) At a pressure of 400 mm Hg and a temperature of 70 degrees C, is each a substance a liquid, a gas or a mixture of liquid and gas?
se the vapour pressure curves in the graph, how can I determine the following: a) What is the vapour pressure of ethanol at 60 degrees C? b) At what temperature does heptane ( C7H16) have a vapour pressure of 500 mm Hg? c) What are the approximate boiling points of each of the 3 substances? d) At a pressure of 400 mm Hg and a temperature of 70 degrees C, is each a substance a liquid, a gas or a mixture of liquid and gas?
Organic And Biological Chemistry
7th Edition
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:STOKER, H. Stephen (howard Stephen)
Chapter1: Saturated Hydrocarbons
Section: Chapter Questions
Problem 1.122EP
Related questions
Question
Use the vapour pressure curves in the graph, how can I determine the following:
a) What is the vapour pressure of ethanol at 60 degrees C?
b) At what temperature does heptane ( C7H16) have a vapour pressure of 500 mm Hg?
c) What are the approximate boiling points of each of the 3 substances?
d) At a pressure of 400 mm Hg and a temperature of 70 degrees C, is each a substance a liquid, a gas or a mixture of liquid and gas?
Transcribed Image Text:900
800
Carbon disulfide
700
600
500
Ethanol
400
Heptane
300
200
100
10
20
30
40
50
60
70
80
90
100
110
Temperature (°C)
Vapor pressure (mm Hg)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning