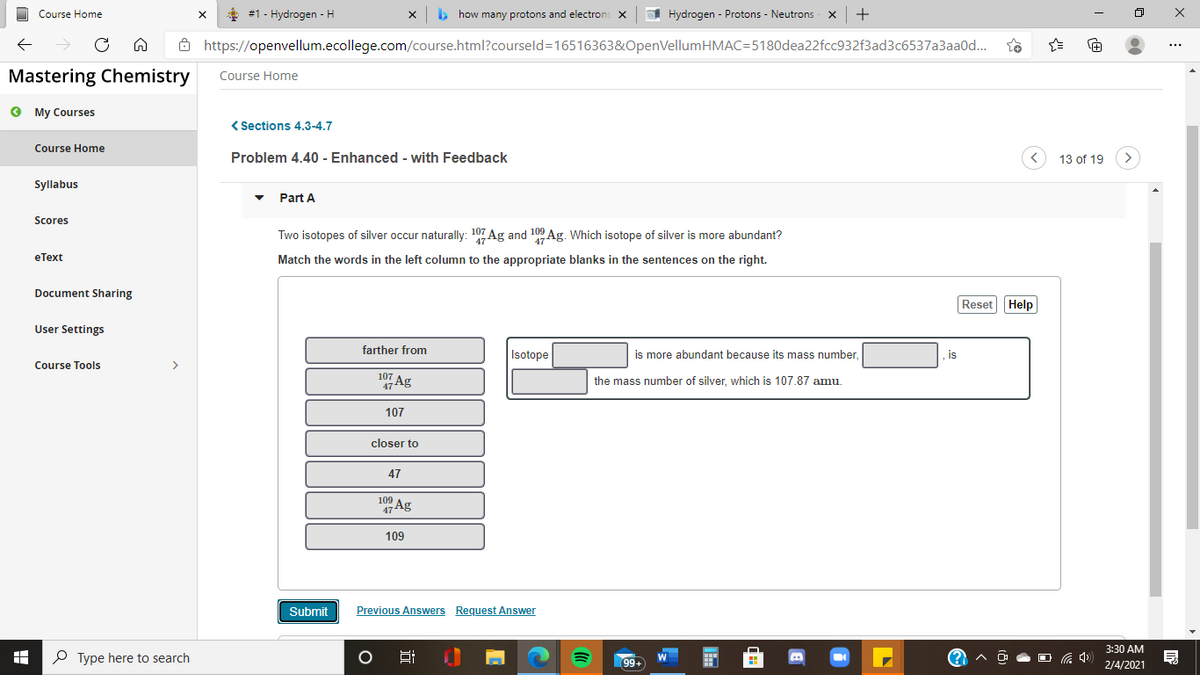
( Sections 4.3-4.7 Problem 4.40 - Enhanced - with Feedback 13 of 19 Part A Two isotopes of silver occur naturally: 107 Ag and 100 Ag. Which isotope of silver is more abundant? Match the words in the left column to the appropriate blanks in the sentences on the right. Reset Help farther from Isotope is more abundant because its mass number, is 107 47 Ag the mass number of silver, which is 107.87 amu. 107 closer to 47 1Ag 109 Submit Previous Answers Request Answer
( Sections 4.3-4.7 Problem 4.40 - Enhanced - with Feedback 13 of 19 Part A Two isotopes of silver occur naturally: 107 Ag and 100 Ag. Which isotope of silver is more abundant? Match the words in the left column to the appropriate blanks in the sentences on the right. Reset Help farther from Isotope is more abundant because its mass number, is 107 47 Ag the mass number of silver, which is 107.87 amu. 107 closer to 47 1Ag 109 Submit Previous Answers Request Answer
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section3.9: Classifying Reactions In Aqueous Solution
Problem 2.2ACP
Related questions
Question
Could someone explain this to me ?
Transcribed Image Text:Course Home
#1 - Hydrogen - H
b how many protons and electron:
A Hydrogen - Protons - Neutrons x
Ô https://openvellum.ecollege.com/course.html?courseld=16516363&OpenVellumHMAC=5180dea22fcc932f3ad3c6537a3aa0d.
Mastering Chemistry
Course Home
O My Courses
< Sections 4.3-4.7
Course Home
Problem 4.40 - Enhanced - with Feedback
13 of 19
Syllabus
Part A
Scores
Two isotopes of silver occur naturally: 107 Ag and 109 Ag. Which isotope of silver is more abundant?
eТеxt
Match the words in the left column to the appropriate blanks in the sentences on the right.
Document Sharing
Reset Help
User Settings
farther from
Isotope
is more abundant because its mass number
is
Course Tools
107
47 Ag
the mass number of silver, which is 107.87 amu.
107
closer to
47
109
47 Ag
109
Submit
Previous Answers Request Answer
3:30 AM
P Type here to search
99+
2/4/2021
Expert Solution
Step 1
Find the solution below
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning