II Review | Constants | Periodic Table The following diagram(Figure 1) is a representation of 20 atoms of a fictitious element, which we will call nevadium (Nv). The red spheres are 293 Nv, Assuming that this sample is a statistically representative sample of the element, calculate the percent abundance of each element. 295 and the blue spheres are Nv. Express your answers as integers. Enter your answers numerically, separated by a comma. Figure 1 of 1 % abundance 293 Nv. % abundance 205 Nv = % Submit Request Answer Part B Complete previous part(s) Provide Feedhack Next
II Review | Constants | Periodic Table The following diagram(Figure 1) is a representation of 20 atoms of a fictitious element, which we will call nevadium (Nv). The red spheres are 293 Nv, Assuming that this sample is a statistically representative sample of the element, calculate the percent abundance of each element. 295 and the blue spheres are Nv. Express your answers as integers. Enter your answers numerically, separated by a comma. Figure 1 of 1 % abundance 293 Nv. % abundance 205 Nv = % Submit Request Answer Part B Complete previous part(s) Provide Feedhack Next
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 25E: The average atomic masses of some elements may vary, depending upon the sources of their ores....
Related questions
Question
Transcribed Image Text:I Review | Constants | Periodic Table
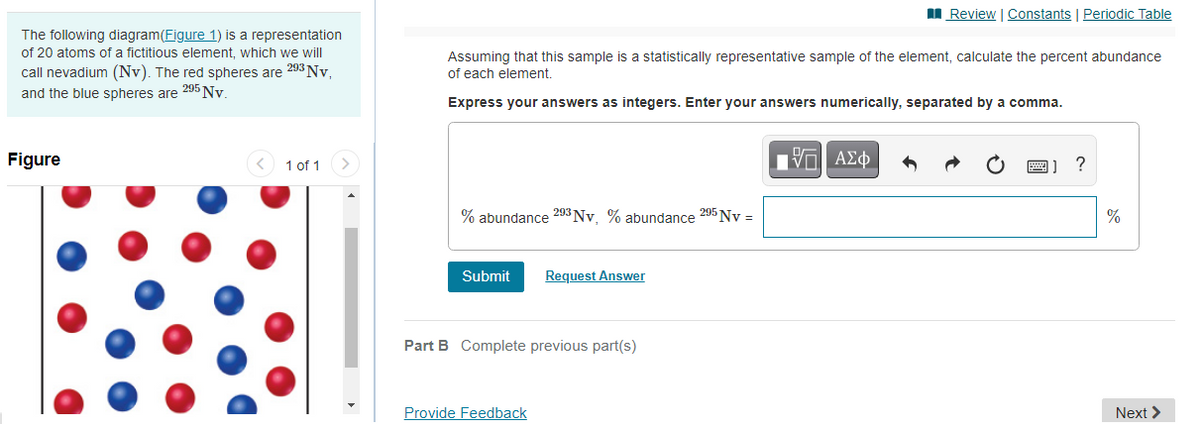
The following diagram(Figure 1) is a representation
of 20 atoms of a fictitious element, which we will
call nevadium (Nv). The red spheres are 203 Nv,
and the blue spheres are 295 Ny
Assuming that this sample is a statistically representative sample of the element, calculate the percent abundance
of each element.
Express your answers as integers. Enter your answers numerically, separated by a comma.
Figure
1 of 1
ΑΣφ
?
% abundance 293 Nv. % abundance 295 Ny =
Submit
Request Answer
Part B Complete previous part(s)
Provide Feedback
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning