Select all the atoms that have at least one lone (unshared) pair of electrons. • Gray = C; white H; red= 0; blue = N; dark green= Cl; brown Br; light green F; purple = 1; yellow S; orange - P • Double click to select atoms. You can zoom in and out using the mouse scroll wheel (or pinch to zoom on touch screens). Convert the model below to a skeletal drawing. wireframe H H H HH H H + labels H H
Select all the atoms that have at least one lone (unshared) pair of electrons. • Gray = C; white H; red= 0; blue = N; dark green= Cl; brown Br; light green F; purple = 1; yellow S; orange - P • Double click to select atoms. You can zoom in and out using the mouse scroll wheel (or pinch to zoom on touch screens). Convert the model below to a skeletal drawing. wireframe H H H HH H H + labels H H
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter13: The Geometrical Structure Of Molecules-an Experiment Using Molecular Models
Section: Chapter Questions
Problem 2ASA: The model consists of balls and sticks. a. How many holes should be in the ball you select for the N...
Related questions
Question
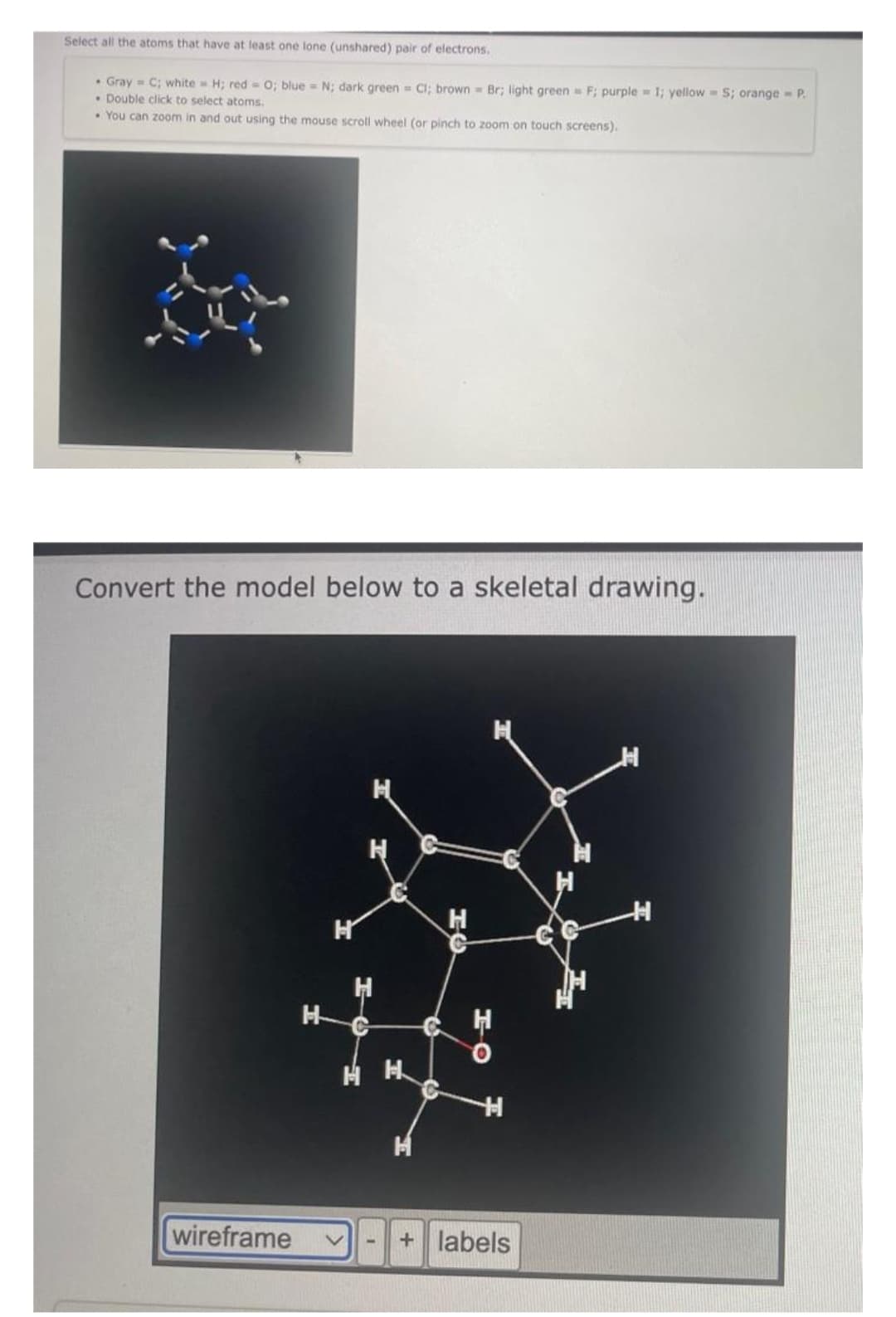
Transcribed Image Text:Select all the atoms that have at least one lone (unshared) pair of electrons.
• Gray = C; white H; red= 0; blue = N; dark green= Cl; brown Br; light green F; purple = 1; yellow=S; orange - P
Double click to select atoms.
You can zoom in and out using the mouse scroll wheel (or pinch to zoom on touch screens).
Convert the model below to a skeletal drawing.
wireframe
H
H
H
C
H
H
+ labels
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
