Show that if it can be assumed that there is a pre-equilibrium involving step v=k₂K[CH₂CICH₂OH][OH-]/ce, (1), the rate of formation of ethylene oxide is v= where K is the equilibrium constant for the first step and k, is the rate constant ons for the second step. d lo notioqu
Show that if it can be assumed that there is a pre-equilibrium involving step v=k₂K[CH₂CICH₂OH][OH-]/ce, (1), the rate of formation of ethylene oxide is v= where K is the equilibrium constant for the first step and k, is the rate constant ons for the second step. d lo notioqu
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter13: Chemical Kinetics
Section: Chapter Questions
Problem 13.49QE: When formic acid is heated, it decomposes to hydrogen and carbon dioxide in a first-order decay....
Related questions
Question
Complete all steps
![Show that if it can be assumed that there is a pre-equilibrium involving step
= k₂K[CH₂CICH₂OH][OH-]/ce,
(1), the rate of formation of ethylene oxide is v=
where K is the equilibrium constant for the first step and k, is the rate constant
for the second
step.
rottsdy 1A for!!
action consists of a pre-equilibrium sten
nog sist art
sbrolib](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fc944e8dc-d275-4b70-8def-6573a43e5eec%2F06674f8b-7ff3-4498-b019-50d6b7ed25a2%2Fyk39lul_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Show that if it can be assumed that there is a pre-equilibrium involving step
= k₂K[CH₂CICH₂OH][OH-]/ce,
(1), the rate of formation of ethylene oxide is v=
where K is the equilibrium constant for the first step and k, is the rate constant
for the second
step.
rottsdy 1A for!!
action consists of a pre-equilibrium sten
nog sist art
sbrolib
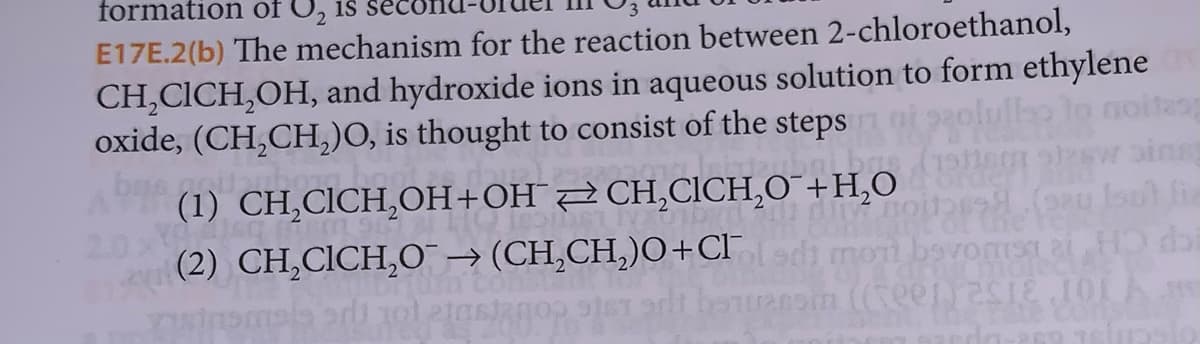
Transcribed Image Text:formation of O₂ is
E17E.2(b) The mechanism for the reaction between 2-chloroethanol,
CH₂CICH₂OH, and hydroxide ions in aqueous solution to form ethylene
oxide, (CH₂CH₂)O, is thought to consist of the steps
szolulls to noites
bas colo
(1) CH₂CICH₂OH+OHCH₂CICH₂O
Yo Lleg
HAHA (19tistor ATLE
+ H₂Orig
2 noitara
(pau Isu) lia
(2) CH₂CICH₂O → (CH₂CH₂)O+CIl sdi mori bevosta HD di
vistasmala od 101 21nstagoo
molect
boranom ((seenesie 101 A
-252 clipalo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax