Show that in isothermal altitude bands where temperature, T, is constant, the pressure, P, varies with the altitude, h, as: P In- RT The atmosphere of Jupiter is essentially made up of hydrogen, H2. For H2, the specific gas constant, R, is 4157 J/(kg K). The acceleration of gravity of Jupiter, g, is 24.9 m/s². Assuming an isothermal atmosphere with a temperature of 150 K and assuming that Jupiter has a definable surface, calculate the altitude above that surface where the pressure is one-half the surface pressure..
Show that in isothermal altitude bands where temperature, T, is constant, the pressure, P, varies with the altitude, h, as: P In- RT The atmosphere of Jupiter is essentially made up of hydrogen, H2. For H2, the specific gas constant, R, is 4157 J/(kg K). The acceleration of gravity of Jupiter, g, is 24.9 m/s². Assuming an isothermal atmosphere with a temperature of 150 K and assuming that Jupiter has a definable surface, calculate the altitude above that surface where the pressure is one-half the surface pressure..
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter5: Analysis Of Convection Heat Transfer
Section: Chapter Questions
Problem 5.13P: 5.13 The torque due to the frictional resistance of the oil film between a rotating shaft and its...
Related questions
Question
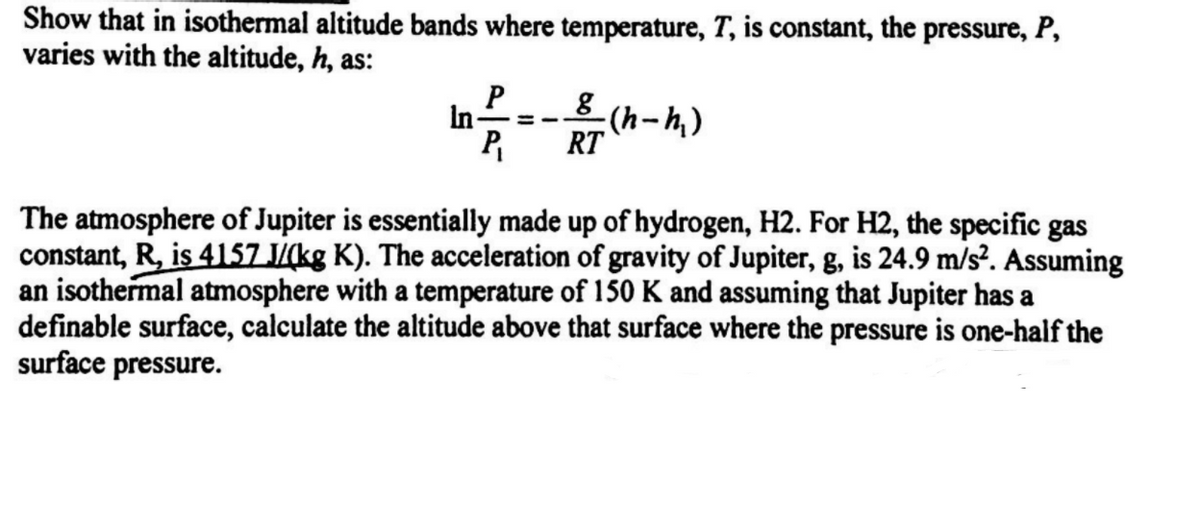
Transcribed Image Text:Show that in isothermal altitude bands where temperature, T, is constant, the pressure, P,
varies with the altitude, h, as:
In-
8 (h-h,)
P
RT
The atmosphere of Jupiter is essentially made up of hydrogen, H2. For H2, the specific gas
constant, R, is 4157 J/(kg K). The acceleration of gravity of Jupiter, g, is 24.9 m/s². Assuming
an isothermal atmosphere with a temperature of 150 K and assuming that Jupiter has a
definable surface, calculate the altitude above that surface where the pressure is one-half the
surface pressure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning