Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 2CQ: Under what circumstances would you expect a gas to behave significantly differently than predicted...
Related questions
Question
need typed
don't copy from others I will downvote for sure
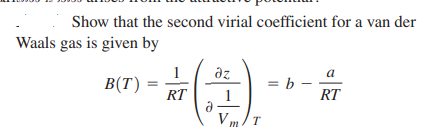
Transcribed Image Text:Show that the second virial coefficient for a van der
Waals gas is given by
1
B(T)
a
az
= b –
RT
RT
V m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Classical Dynamics of Particles and Systems
Physics
ISBN:
9780534408961
Author:
Stephen T. Thornton, Jerry B. Marion
Publisher:
Cengage Learning


Classical Dynamics of Particles and Systems
Physics
ISBN:
9780534408961
Author:
Stephen T. Thornton, Jerry B. Marion
Publisher:
Cengage Learning