The p-Vdiagram in the figure shows two paths along which a sample of gas can be taken from state a to state b. where V 8.0V, Path 1 requires that energy equal to 17.50p,Vi be transferred to the gas as heat. Path 2 requires that energy equal to 21.50PV, be transferred to the eas ash What is th
The p-Vdiagram in the figure shows two paths along which a sample of gas can be taken from state a to state b. where V 8.0V, Path 1 requires that energy equal to 17.50p,Vi be transferred to the gas as heat. Path 2 requires that energy equal to 21.50PV, be transferred to the eas ash What is th
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 31P: A dilute gas at a pressure of 2.0 atm and a volume of 4.0 L is taken through the following...
Related questions
Question
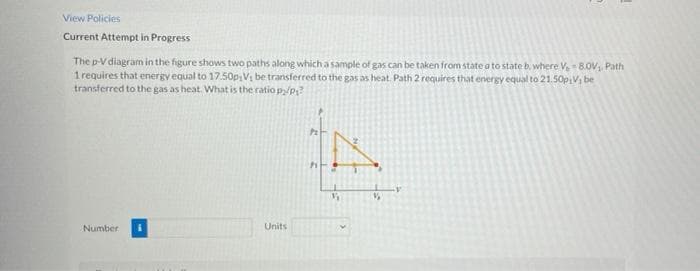
Transcribed Image Text:View Policies
Current Attempt in Progress
The p-V diagram in the figure shows two paths along which a sample of gas can be taken from state a to state b, where V 8.0V, Path
1 requires that energy equal to 17.50psVị be transferred to the gas as heat. Path 2 requires that energy equal to 21.50p Vi be
transferred to the gas as heat. What is the ratio py/p?
Number
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

