Show the reaction and equilibrium expression for the basic dissociation of trimethylamine. Given that the Ka = 1.58 x 10-10 ammonium ion, use this to calculate the equilibrium constant for the basic dissociation of trimethyl amine. If the concentration of trimethylamine is 0.10 M, what is the pH of the solution? for trimethyl
Show the reaction and equilibrium expression for the basic dissociation of trimethylamine. Given that the Ka = 1.58 x 10-10 ammonium ion, use this to calculate the equilibrium constant for the basic dissociation of trimethyl amine. If the concentration of trimethylamine is 0.10 M, what is the pH of the solution? for trimethyl
Chapter20: Carboxylic Acids And Nitriles
Section20.4: Substituent Effects On Acidity
Problem 7P: Dicarboxylic acids have two dissociation constants, one for the initial dissociation into a...
Related questions
Question
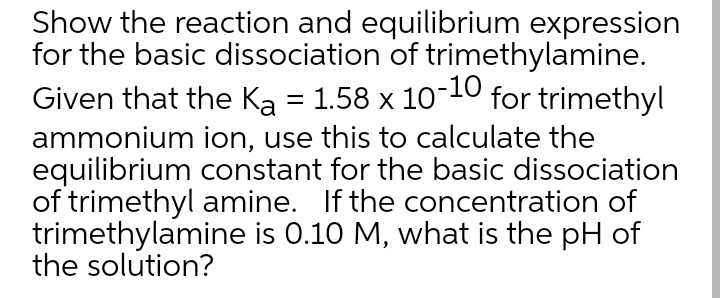
Transcribed Image Text:Show the reaction and equilibrium expression
for the basic dissociation of trimethylamine.
-10
Given that the Ka = 1.58 x 10 for trimethyl
ammonium ion, use this to calculate the
equilibrium constant for the basic dissociation
of trimethyl amine. If the concentration of
trimethylamine is 0.10 M, what is the pH of
the solution?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



